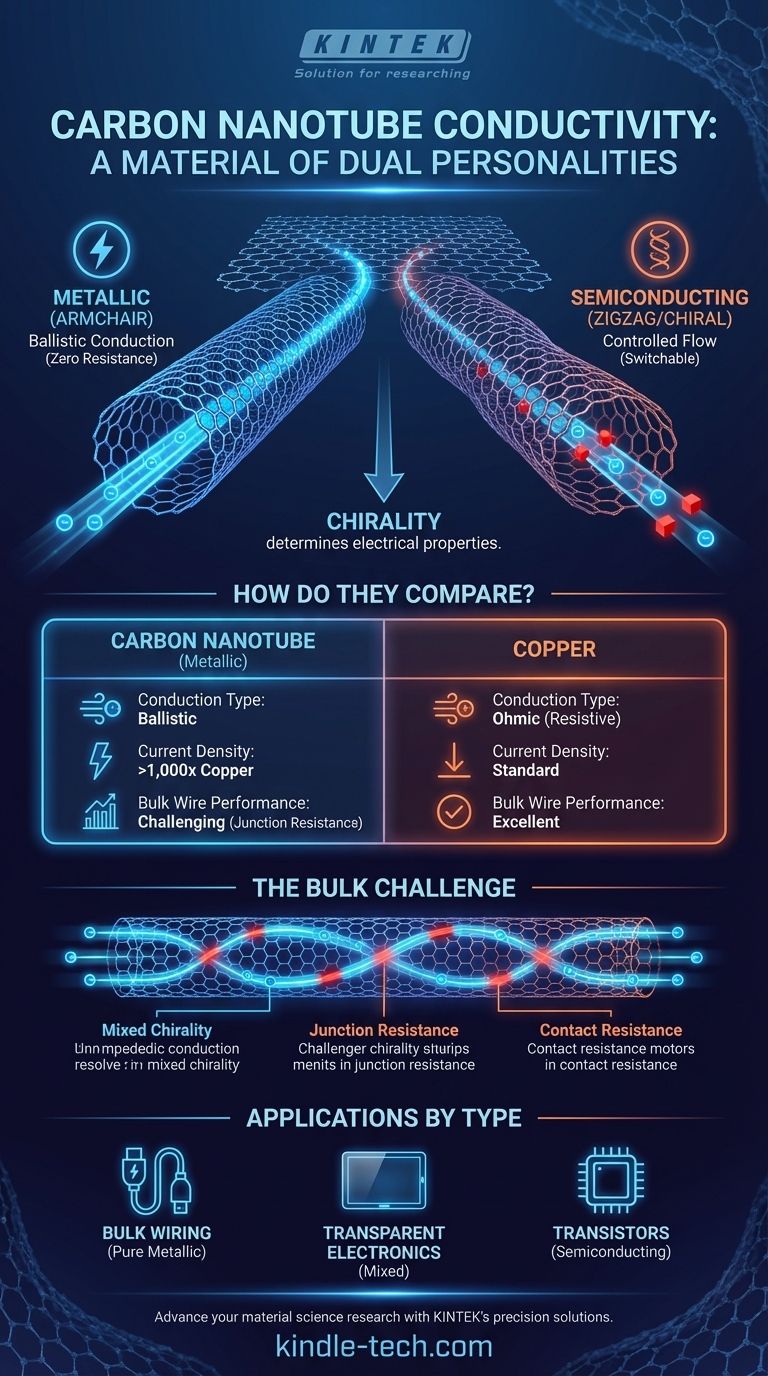
When it comes to conductivity, a carbon nanotube is a material of dual personalities. Yes, an individual carbon nanotube can be an extraordinary conductor of electricity, exhibiting properties far superior to traditional metals like copper. However, its actual performance is entirely dictated by its specific atomic structure, meaning some nanotubes are perfect metallic conductors while others behave like semiconductors.
The central takeaway is that a carbon nanotube's electrical properties are not fixed. They are determined by its chirality—the specific angle of its atomic lattice. This structural dependency is both the source of its incredible potential and the primary challenge to its widespread use as a bulk conductor.

The Heart of Conductivity: Chirality and Structure
To understand why carbon nanotubes (CNTs) behave this way, we must look at how they are formed and how their atomic arrangement dictates the flow of electrons.
From Graphene to Nanotube
A carbon nanotube is best visualized as a single sheet of graphene (a one-atom-thick layer of carbon atoms in a honeycomb pattern) that has been seamlessly rolled into a cylinder. The incredible electrical properties of graphene are the foundation for the nanotube's potential.
The Concept of Chirality
The way this graphene sheet is "rolled" determines everything. This angle of rolling is known as chirality.
Imagine a piece of paper with a honeycomb pattern printed on it. You can roll it in different ways: perfectly straight, at a slight angle, or sideways. Each method would create a different alignment of the honeycomb pattern along the tube's seam, and this is precisely what happens at the atomic scale with CNTs.
Armchair vs. Zigzag and Chiral
The specific geometry, defined by a pair of indices (n,m), sorts CNTs into three main types.
- Armchair: These nanotubes are rolled in a way that their structure is perfectly symmetrical along the axis. Armchair nanotubes are always metallic and are exceptional conductors.
- Zigzag and Chiral: These nanotubes are rolled at different angles. Depending on the specific angle, these tubes can be either metallic or semiconducting. Statistically, about one-third of these are metallic, and two-thirds are semiconducting.
How Do They Compare to Traditional Conductors?
An individual, metallic carbon nanotube isn't just a good conductor; it operates on a different level than materials like copper or silver due to quantum mechanical effects.
Ballistic Conduction
At microscopic lengths, electrons can pass through a perfect metallic nanotube without scattering off atoms and losing energy as heat. This phenomenon, known as ballistic conduction, means the nanotube has virtually zero electrical resistance.
Current-Carrying Capacity
Thanks to the immense strength of the carbon-carbon atomic bonds, CNTs have an astonishingly high current-carrying capacity (ampacity). They can handle current densities more than 1,000 times greater than copper without degrading or melting.
The Bulk Material Challenge
The exceptional properties described above apply to single, perfect nanotubes. However, a real-world wire is made of trillions of nanotubes bundled together. This introduces significant challenges that diminish the overall performance of the bulk material.
Understanding the Trade-offs and Practical Hurdles
The transition from a single nanotube's theoretical potential to a functional, macroscopic wire is fraught with engineering obstacles.
The Chirality Control Problem
Current manufacturing methods produce a mixture of metallic and semiconducting nanotubes. The presence of semiconducting tubes in a wire intended for conduction severely impedes electron flow, acting like roadblocks for electricity. Separating them is a complex and costly process.
Junction Resistance
In a CNT wire, electrons must constantly hop from one nanotube to the next. Each junction between tubes creates a point of resistance. The cumulative effect of these trillions of junctions is the primary reason that current CNT wires often underperform compared to copper.
Contact Resistance
Simply getting electricity from a conventional metal wire into the carbon nanotube material efficiently is another significant challenge. The connection point, or contact, creates its own resistance that must be minimized for high-performance applications.
Making the Right Choice for Your Goal
Whether a carbon nanotube is a "good conductor" depends entirely on the context of your application. The very properties that make it difficult for one use case make it ideal for another.
- If your primary focus is replacing bulk wiring like copper: You must prioritize purity and alignment. The goal is to maximize the number of metallic nanotubes and minimize junction resistance, a significant challenge that currently limits their widespread use in this area.
- If your primary focus is creating transparent electronics: A random network of mixed-chirality CNTs is ideal. Such films are conductive enough for touch screens or solar cells, and their semiconducting properties are not a major drawback.
- If your primary focus is developing next-generation transistors: You must isolate and use only the semiconducting nanotubes. Here, the goal is to leverage their ability to switch on and off, which is the foundation of computer logic.
Ultimately, the exceptional conductivity of a carbon nanotube is a precise property unlocked only when its specific atomic structure is matched to the demands of the application.
Summary Table:
| Property | Carbon Nanotube (Metallic) | Copper |
|---|---|---|
| Conduction Type | Ballistic (low resistance) | Ohmic (resistive) |
| Current Density | >1,000x higher | Standard |
| Bulk Wire Performance | Challenging (junction resistance) | Excellent |
| Primary Use Case | Nanoscale electronics, specialized applications | General wiring |
Ready to integrate cutting-edge materials like carbon nanotubes into your research? KINTEK specializes in providing high-precision lab equipment and consumables tailored for advanced material science. Whether you're developing next-generation electronics or conducting nanoscale research, our solutions ensure accuracy and reliability. Contact our experts today to discuss how we can support your laboratory's specific needs and help you achieve breakthrough results.
Visual Guide
Related Products
- Conductive Carbon Cloth Carbon Paper Carbon Felt for Electrodes and Batteries
- Conductive Boron Nitride BN Ceramics Composite for Advanced Applications
- Laboratory Hydraulic Press Lab Pellet Press for Button Battery
- Laboratory CVD Boron Doped Diamond Materials
- Graphite Vacuum Continuous Graphitization Furnace
People Also Ask
- What are the three types of coating? A Guide to Architectural, Industrial, and Special Purpose
- Why are high surface area materials preferred for BES anodes? Maximize Microbial Power and Efficiency
- What are the material properties of carbon paper? Unlocking High Conductivity & Porosity for Your Lab
- What is the ideal operating environment for a glassy carbon sheet? Ensure Optimal Performance and Longevity
- What are the four main types of sensors? A Guide to Power Source and Signal Type



















