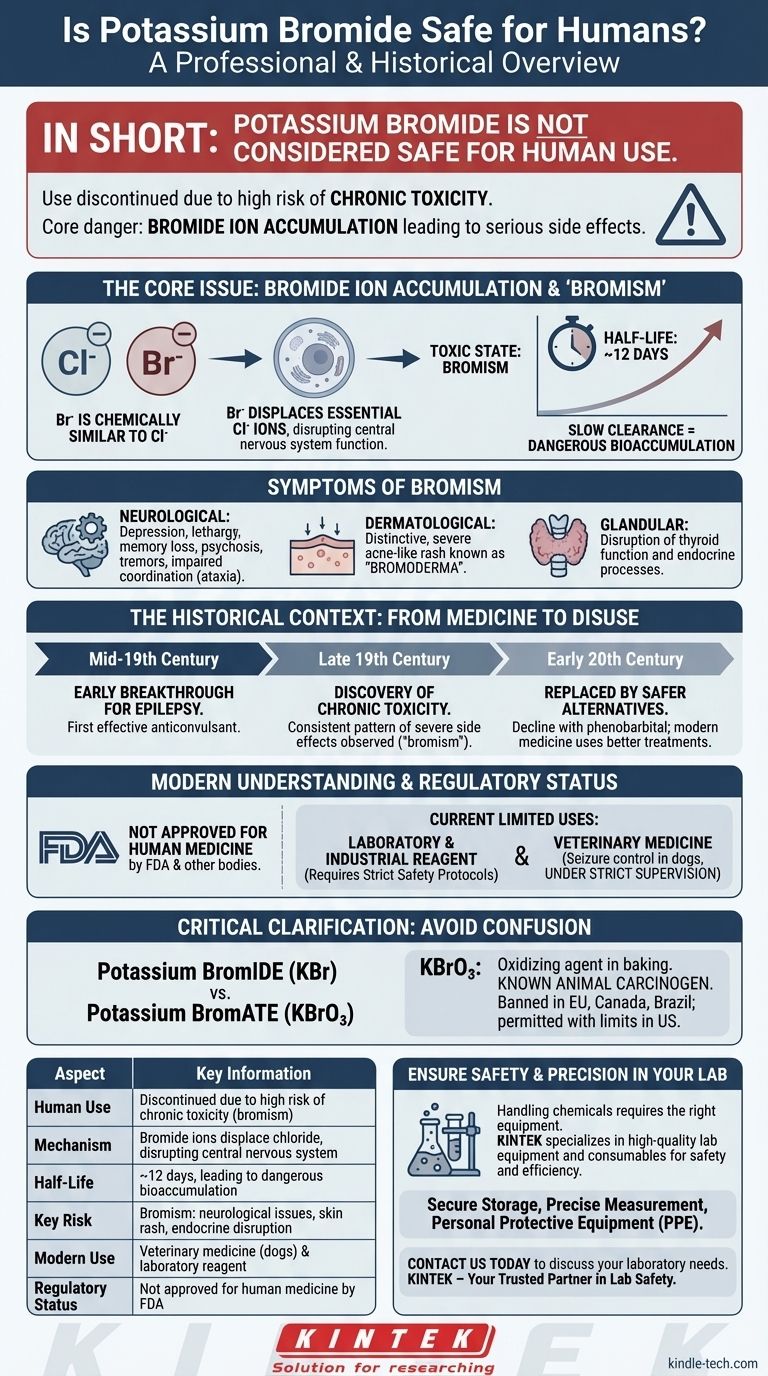
In short, potassium bromide is not considered safe for human use. While it was a common prescription medication over a century ago for conditions like epilepsy, its use in humans has been discontinued in most countries due to a high risk of chronic toxicity. The core danger lies in the bromide ion, which can accumulate in the body over time and lead to serious neurological and physiological side effects.
The central issue with potassium bromide is the accumulation of bromide ions in the body, which can displace essential chloride ions and disrupt the central nervous system. This leads to a toxic state known as "bromism," making it an unacceptable risk for human treatment given the availability of far safer alternatives.

The Historical Context: From Medicine to Disuse
Potassium bromide holds a significant place in medical history, but our understanding of its risks has evolved dramatically.
An Early Breakthrough for Epilepsy
In the mid-19th century, potassium bromide was discovered to be one of the first effective treatments for epilepsy. For decades, it was the primary anticonvulsant available, offering relief to patients who previously had no viable options.
The Discovery of Chronic Toxicity
Over time, physicians observed that patients on long-term potassium bromide therapy developed a consistent pattern of severe side effects. This condition, named bromism, highlighted the dangers of chronic exposure.
Why It Was Replaced
The development of safer and more effective anticonvulsants, such as phenobarbital in the early 20th century, began the decline of potassium bromide's use in humans. Today, modern medicine relies on a range of treatments with much better safety profiles.
How Potassium Bromide Affects the Body
The toxicity of potassium bromide is not immediate like a classic poison but is a result of its slow, cumulative effect on fundamental body chemistry.
The Mechanism of Bromide Toxicity
The bromide ion (Br⁻) is chemically very similar to the chloride ion (Cl⁻), which is essential for nerve function and fluid balance. When ingested, bromide ions compete with and displace chloride ions in the body's cells, particularly in the brain.
This interference disrupts the normal function of neurotransmitters like GABA, leading to its sedative and anticonvulsant effects, but also its widespread toxicity.
The Danger of Bioaccumulation
Potassium bromide has an extremely long half-life of about 12 days in humans. This means it is cleared from the body very slowly.
With repeated doses, the bromide level builds steadily, making it easy to reach a toxic concentration even on what might seem like a low dose.
Symptoms of "Bromism"
Chronic exposure leads to a multi-system disorder with a wide range of symptoms, including:
- Neurological: Depression, lethargy, memory loss, psychosis, tremors, and impaired coordination (ataxia).
- Dermatological: A distinctive and severe acne-like rash known as bromoderma.
- Glandular: Disruption of thyroid function and other endocrine processes.
Understanding the Modern Context
While it is no longer used for humans, potassium bromide still exists in specific, controlled applications. It is also frequently confused with a different compound.
Current Regulatory Status
The U.S. Food and Drug Administration (FDA) and equivalent bodies in most other countries have removed potassium bromide from approved use in human medicine. It is now primarily a chemical reagent used in laboratory and industrial settings, where safety protocols are required for handling.
The Exception: Veterinary Medicine
Potassium bromide is still used in veterinary medicine, almost exclusively to control seizures in dogs that do not respond to more modern medications.
This treatment requires strict veterinary supervision, including regular blood tests to monitor bromide levels and manage the inevitable side effects. Its use in animals is a carefully weighed trade-off between seizure control and toxicity.
The Confusion with Potassium Bromate
It is critical not to confuse potassium bromide (KBr) with potassium bromate (KBrO₃).
Potassium bromate is an oxidizing agent sometimes used to strengthen dough in commercial baking. It is a known animal carcinogen and is classified as a possible human carcinogen. For this reason, its use in food products is banned in the European Union, Canada, Brazil, and many other countries, though it remains permitted under specific limits in the U.S.
Making the Right Choice for Your Goal
Your approach to potassium bromide should be guided by your specific context and a clear understanding of its risks.
- If your primary focus is personal health: Avoid any product or old remedy containing potassium bromide. It is unequivocally unsafe for human self-administration.
- If your primary focus is food safety: Your concern is likely with potassium bromate (KBrO₃), not bromide. Check food labels on baked goods and research the regulations in your country.
- If your primary focus is veterinary care: Only administer potassium bromide to your pet under the direct and continuous supervision of a qualified veterinarian who can monitor its effects and toxicity.
- If your primary focus is laboratory safety: Treat potassium bromide as a hazardous chemical. Always consult the Safety Data Sheet (SDS) and use appropriate personal protective equipment (PPE) like gloves and goggles.
Ultimately, understanding the history and science of potassium bromide confirms why this once-useful drug no longer meets modern standards of safety for human medicine.
Summary Table:
| Aspect | Key Information |
|---|---|
| Human Use | Discontinued due to high risk of chronic toxicity (bromism) |
| Mechanism | Bromide ions displace chloride, disrupting the central nervous system |
| Half-Life | ~12 days, leading to dangerous bioaccumulation |
| Key Risk | Bromism: neurological issues, skin rash (bromoderma), endocrine disruption |
| Modern Use | Veterinary medicine (for dogs) & laboratory reagent |
| Regulatory Status | Not approved for human medicine by FDA and other agencies |
Ensure Safety and Precision in Your Lab
Handling chemicals like potassium bromide requires the right equipment and consumables to maintain a safe and efficient workflow. KINTEK specializes in providing high-quality lab equipment and consumables tailored to meet the rigorous demands of modern laboratories.
Whether you need secure storage solutions, precise measurement tools, or personal protective equipment (PPE), we have the products to support your safety protocols and research goals. Let our expertise help you create a safer lab environment.
Contact us today to discuss your laboratory needs and discover how KINTEK can be your trusted partner in lab safety and efficiency.
Visual Guide
Related Products
- Laboratory Jaw Crusher
- Custom PTFE Teflon Parts Manufacturer Corrosion Resistant Cleaning Rack Flower Basket
- Desktop Fast Laboratory Autoclave Sterilizer 35L 50L 90L for Lab Use
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Variable Speed Peristaltic Pump
People Also Ask
- What does the centrifuge do to the solution? Separate Components with High-Speed Centrifugal Force
- What is the sizing process in sintering? Master Dimensional Control for Precision Parts
- At what temperature will quartz melt? Unlocking Its Complex High-Temperature Journey
- What are the different types of biomass pellets? A Guide to Wood vs. Agricultural Pellets
- What are the different types of heat treatment furnaces? Choose the Right Furnace for Your Material's Success
- What are the important parameters which affect the sputtering process? Master Thin Film Deposition Control
- What materials can be used in sintering? Explore Metals, Ceramics & Composites
- What is the process of thin film production? A Guide to Atomic-Level Material Engineering















