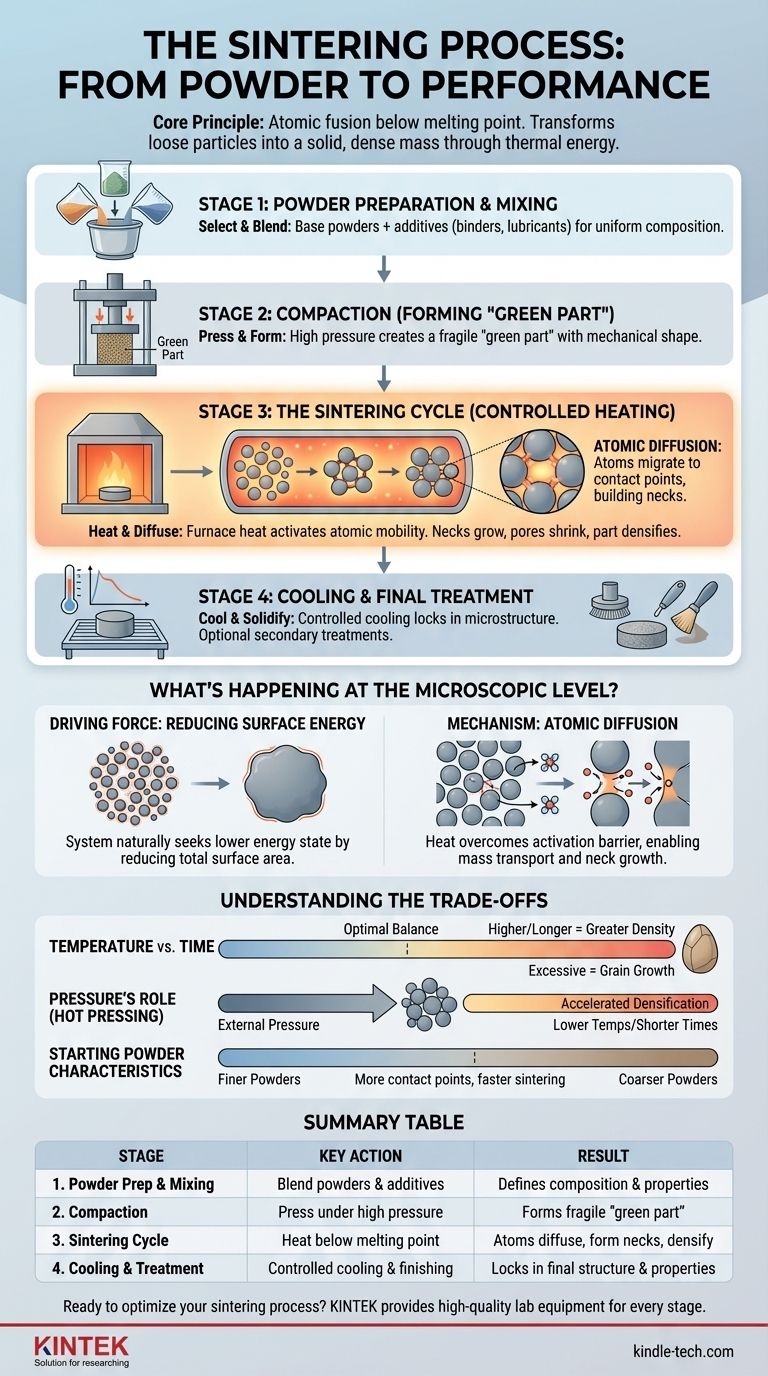
At its core, the sintering process consists of three primary stages: preparing and mixing a base powder, compacting that powder into a preliminary shape, and applying controlled heat to fuse the particles into a solid, dense object. This thermal treatment happens below the material's melting point, relying on atomic diffusion to bond the particles and remove the voids between them.
Sintering is not a process of melting, but of atomic fusion. It transforms a loose collection of particles into a strong, solid mass by using thermal energy to encourage atoms to diffuse across particle boundaries, effectively welding the material together from the inside out.

The Sintering Framework: From Powder to Part
The journey from a raw powder to a finished component is a precise, multi-stage process. Each step builds upon the last, with the initial preparation having a significant impact on the final product's quality and properties.
Stage 1: Powder Preparation and Mixing
This is the foundational stage where the final material's properties are defined. The process begins by selecting and preparing the raw material, which is typically a fine powder of metal, ceramic, or another substance.
These powders are then precisely mixed to achieve the desired chemical composition. Additives like binders (for initial strength), lubricants (to aid in compaction), or alloying elements (to enhance properties) may be introduced to create a uniform, homogenous blend.
Stage 2: Compaction (Forming the "Green Part")
The mixed powder is loaded into a die and subjected to high pressure. This step, known as compaction, presses the particles together, mechanically densifying the material and forming it into the desired shape.
The resulting object is called a "green part." It is fragile and has only enough structural integrity to be handled, but this step is critical for eliminating large voids and ensuring uniform density before heating.
Stage 3: The Sintering Cycle (Controlled Heating)
The green part is placed into a sintering furnace where it undergoes a carefully controlled thermal cycle. This is the heart of the process, where the real transformation occurs.
The temperature is raised to a point below the material's melting point. At this elevated temperature, the atoms in the particles become highly mobile. They begin to diffuse across the boundaries of adjacent particles, forming small bridges or "necks."
As the heating continues, these necks grow wider, pulling the particles closer together. This action shrinks the pores between the particles, causing the entire part to densify, gain significant strength, and shrink in overall size.
Stage 4: Cooling and Final Treatment
After holding at the sintering temperature for a specific duration, the part is cooled in a controlled manner. This prevents thermal shock, which could cause cracks, and helps lock in the desired final microstructure and mechanical properties.
Depending on the application, sintered parts may undergo secondary treatments like machining, coating, or heat treatment to meet final specifications.
What's Happening at the Microscopic Level?
To truly master the process, you must understand the physics driving the transformation. Sintering is governed by fundamental principles of thermodynamics and atomic movement.
The Driving Force: Reducing Surface Energy
A fine powder has an enormous amount of surface area relative to its volume. From a thermodynamic perspective, this high surface area represents a state of high surface energy.
The universe favors lower energy states. The sintering process is a natural pathway to reduce this excess energy by eliminating the surfaces between particles and forming a single, solid mass with less total surface area.
The Mechanism: Atomic Diffusion
Heat provides the energy needed to overcome the activation barrier for atomic diffusion. Atoms migrate from the bulk of the particles to the points of contact, building the "necks" that bridge the gap between them.
This mass transport continues over time, growing the necks and pulling the centers of the particles closer. The result is the elimination of porosity and an increase in the part's overall density and strength.
Understanding the Trade-offs
Sintering is a balancing act between competing variables. Controlling these factors is key to achieving consistent and predictable results.
Temperature vs. Time
The two most critical parameters are sintering temperature and time. Higher temperatures or longer hold times generally lead to greater densification and strength.
However, excessive heat or time can cause grain growth, where smaller crystal grains merge into larger ones. While the part becomes dense, large grains can sometimes reduce toughness and other mechanical properties. The goal is to find the optimal balance for the specific material and application.
Pressure's Role (Hot Pressing)
For materials that are difficult to sinter, such as tungsten or certain advanced ceramics, external pressure can be applied during the heating cycle. This process, known as hot pressing, physically forces the particles closer together, accelerating diffusion and enabling densification at lower temperatures or in shorter times.
Starting Powder Characteristics
The final product is highly dependent on the initial powder. Finer powders have more surface area and contact points, meaning they possess a stronger driving force for sintering. They typically sinter faster and at lower temperatures than coarser powders.
Making the Right Choice for Your Goal
The specific parameters of the sintering process should be tailored to your primary objective.
- If your primary focus is achieving maximum density and strength: Use very fine starting powders and an optimized heating cycle, and consider pressure-assisted sintering (hot pressing) for superior results.
- If your primary focus is creating parts with controlled porosity (e.g., for filters): Use larger, more uniform particles and intentionally shorten the sintering time or lower the temperature to fuse the particles without fully eliminating the voids.
- If your primary focus is cost-effective, high-volume production: Standardize on a repeatable cold compaction and furnace sintering process, ensuring tight control over raw material consistency and furnace parameters.
Understanding these fundamental stages empowers you to manipulate material properties at the atomic level, turning simple powders into high-performance components.
Summary Table:
| Stage | Key Action | Result |
|---|---|---|
| 1. Powder Prep & Mixing | Select and blend base powders with additives. | Defines final material composition and properties. |
| 2. Compaction | Press powder in a die under high pressure. | Forms a fragile "green part" in the desired shape. |
| 3. Sintering Cycle | Heat the green part below its melting point. | Atoms diffuse, forming necks and densifying the part. |
| 4. Cooling & Treatment | Controlled cooling and optional finishing. | Locks in final microstructure and properties. |
Ready to optimize your sintering process for superior material performance?
KINTEK specializes in providing the high-quality lab equipment and consumables essential for every stage of sintering—from reliable powder mixing tools to precision-controlled sintering furnaces. Our expertise helps you achieve consistent density, strength, and complex geometries in your metal or ceramic components.
Contact our sintering experts today to discuss how our solutions can enhance your research and production outcomes.
Visual Guide
Related Products
- 1800℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
- Multi-zone Laboratory Tube Furnace
People Also Ask
- What is the use of muffle furnace in chemistry laboratory? Achieve Precise High-Temperature Material Processing
- Is a muffle furnace an oven? A Guide to High-Temperature vs. Low-Temperature Heating
- What is the introduction of muffle furnace? A Guide to High-Temperature, Contamination-Free Heating
- What is the difference between muffle furnace and hot air oven? Choose the Right Heating Tool for Your Lab
- What is a furnace used in the lab? Your Guide to High-Temperature Precision



















