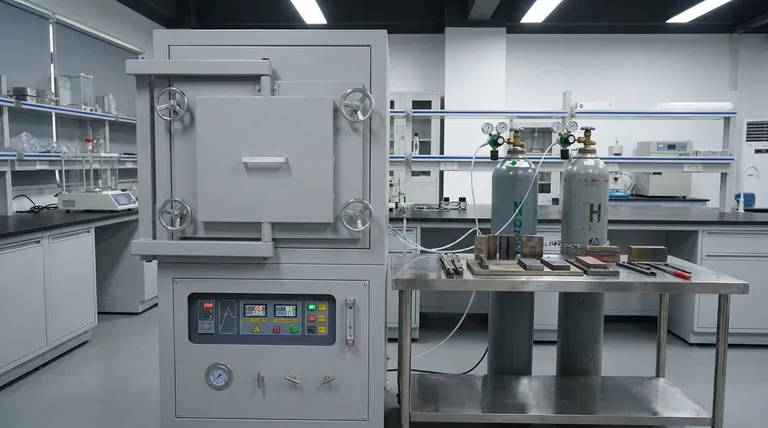
The atmosphere inside a heat treating furnace is a precisely controlled gas mixture designed to interact with the metal being processed. Rather than a single gas, there are several distinct categories of atmospheres used. The most common are categorized as oxidizing (air), inert (argon, nitrogen), reducing (containing hydrogen or carbon monoxide), or a vacuum, each chosen to achieve a specific outcome on the material's surface.
The choice of a furnace atmosphere is not merely about heating; it is about actively controlling the chemical reactions on the metal's surface. The goal is to either protect the material from change or to intentionally alter its surface properties in a predictable way.
Why a Controlled Atmosphere is Critical
Simply heating metal in ambient air can cause undesirable changes. A controlled atmosphere gives engineers precise command over the material's surface chemistry during the high-temperature cycle.
Preventing Unwanted Reactions
The most common reason to use a controlled atmosphere is to protect the part. At high temperatures, oxygen in the air will readily cause oxidation (scaling) and decarburization (loss of carbon from the surface of steel), which can ruin the component's properties.
Inducing Desired Reactions
Conversely, some processes use the atmosphere to intentionally introduce elements into the metal's surface. Processes like carburizing use a carbon-rich atmosphere to harden the surface of steel components, a technique known as case hardening.
Common Furnace Atmospheres and Their Purpose
Each type of atmosphere serves a distinct engineering function. The selection depends entirely on the material being treated and the desired final properties.
Oxidizing Atmospheres (Air)
This is the simplest and most common atmosphere—it is simply ambient air. It is used when a surface oxide layer is either acceptable, desired for appearance, or will be removed by subsequent machining operations.
Inert Atmospheres
Inert gases are used for protection. They work by displacing the oxygen in the furnace, preventing oxidation and other unwanted reactions from occurring on the part's surface.
The most common inert gases are nitrogen and argon. They do not react with the metal, ensuring the surface finish and composition remain unchanged during heating.
Reducing Atmospheres
Reducing atmospheres go a step beyond inert gases. They not only prevent oxidation but can also actively remove existing light oxides from the surface of the part.
These atmospheres typically contain gases like hydrogen or carbon monoxide, which react with and strip oxygen atoms from the metal's surface, leaving it clean and bright.
Vacuum
A vacuum is the ultimate protective atmosphere. By removing virtually all gas molecules from the heating chamber, it eliminates the possibility of gas-metal reactions.
This is critical for highly reactive or sensitive materials, such as titanium or certain superalloys, where even trace amounts of gas could cause contamination and compromise material properties.
Reactive Atmospheres
These atmospheres are designed to intentionally change the surface chemistry of a part. They contain specific elements that diffuse into the metal at high temperatures.
Common examples include carburizing (adding carbon) and nitriding (adding nitrogen) atmospheres. These processes create a very hard, wear-resistant "case" on the surface of a component while leaving the core tougher and more ductile.
Understanding the Trade-offs and System Requirements
Choosing a controlled atmosphere is not without its challenges. The complexity and cost of the furnace system increase significantly when moving away from a simple air atmosphere.
The Challenge of Purity and Sealing
Controlled atmosphere furnaces require extremely high sealing integrity. Any leak can introduce oxygen and moisture from the outside air, contaminating the atmosphere and ruining the process.
The Cost of Complexity
Generating or purchasing specialized gases, along with the vacuum pumps, sensors, and control logic required to manage them, adds significant capital and operational cost. Furnaces must also be built with appropriate materials, like anti-carburizing bricks, to withstand the specific atmosphere.
Safety and Handling
Many atmospheres involve significant safety risks. Hydrogen is highly flammable and explosive, while nitrogen and argon are simple asphyxiants. These systems require fire curtains, explosion-proof devices, and rigorous safety protocols.
Selecting the Right Atmosphere for Your Goal
Your choice must be driven by the technical requirements of the component you are heat treating.
- If your primary focus is simple heating with no surface requirements: An oxidizing (air) atmosphere is the most direct and cost-effective choice.
- If your primary focus is preventing oxidation and discoloration: An inert atmosphere like nitrogen or argon is your standard protective solution.
- If your primary focus is surface hardening and wear resistance: A reactive atmosphere for carburizing or nitriding is necessary to achieve the desired case properties.
- If your primary focus is processing highly reactive or sensitive materials: A vacuum provides the highest level of protection from contamination and unwanted chemical reactions.
Ultimately, mastering furnace atmospheres transforms heat treatment from a simple heating process into a precise, value-adding engineering discipline.
Summary Table:
| Atmosphere Type | Key Gases/Environment | Primary Purpose |
|---|---|---|
| Oxidizing | Air | Heating where surface oxidation is acceptable |
| Inert | Nitrogen, Argon | Protect surfaces from oxidation and discoloration |
| Reducing | Hydrogen, Carbon Monoxide | Remove existing oxides for a bright, clean finish |
| Vacuum | Near-total gas removal | Ultimate protection for reactive/sensitive materials |
| Reactive | Carbon-rich or Nitrogen-rich | Surface hardening via carburizing or nitriding |
Ready to Achieve Precise Heat Treatment Results?
Selecting and maintaining the correct furnace atmosphere is critical to your success. The experts at KINTEK are here to help. We specialize in providing the right lab equipment and consumables to meet your specific heat treating challenges, whether you require a standard protective atmosphere or a complex reactive system.
We can help you:
- Prevent scaling and decarburization to protect your valuable components.
- Achieve perfect case hardening for superior wear resistance.
- Safely process sensitive materials with reliable vacuum or inert gas systems.
Don't let atmosphere control be a bottleneck. Contact our team today for a consultation to ensure your furnace system is optimized for your materials and goals.
Contact KINTEK today to discuss your laboratory's heat treatment needs.
Visual Guide
Related Products
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
- Vertical Laboratory Tube Furnace
People Also Ask
- Why is a vacuum or atmosphere furnace required for SiBCN pyrolysis? Mastering Precision for Superior Ceramics
- What sintering temperatures may be required for tungsten in a pure hydrogen atmosphere? Reach 1600°C for Peak Performance
- Which type of furnace is used in annealing process? Choose the Right Controlled Atmosphere Furnace
- How does argon remove oxygen? By Physically Displacing It to Create an Inert Shield
- What is the function of a high-temperature atmosphere tube furnace in P-NCS synthesis? Expert Insights
- What are the primary functions of a high-temperature atmosphere muffle furnace in Fischer-Tropsch Synthesis?
- What does inerting a space mean? A Guide to Eliminating Fire and Explosion Risks
- What is used as an inert atmosphere? Master Process Control with Nitrogen, Argon & More



















