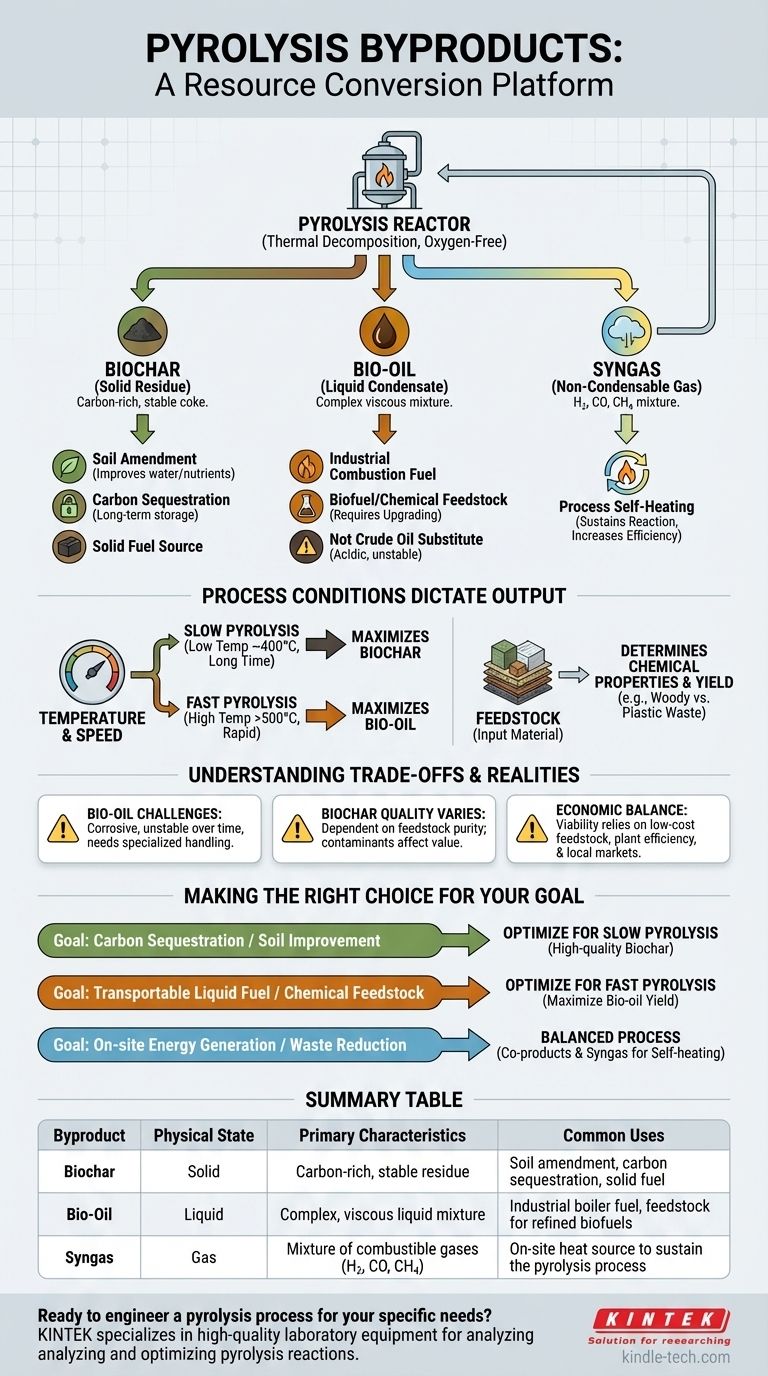
In any pyrolysis reaction, the thermal decomposition of material in an oxygen-free environment consistently yields three distinct categories of byproducts. These are a carbon-rich solid residue known as biochar or coke, a complex liquid mixture called bio-oil or pyrolytic oil, and a blend of non-condensable gases often referred to as syngas. The exact proportion and chemical makeup of these products are not random; they are directly controlled by the input material and the specific conditions of the process.
The critical insight is that pyrolysis is not simply a disposal method, but a highly tunable resource conversion platform. By adjusting the feedstock, temperature, and reaction time, you can deliberately shift the output to favor the production of either solids, liquids, or gases to meet a specific goal.

Deconstructing the Three Core Products
Every pyrolysis operation, regardless of scale or feedstock, will generate outputs in three distinct physical states: solid, liquid, and gas. Understanding the nature and potential use of each is fundamental to evaluating the process.
The Solid Residue: Biochar
Biochar is the stable, carbon-dense solid that remains after the volatile components of the feedstock have vaporized. It is the "charcoal" of the process.
This material is far from being a simple waste product. Its primary applications include its use as a powerful soil amendment in agriculture, where it improves water retention and nutrient stability, and as a method for long-term carbon sequestration.
It can also be processed into activated carbon for filtration or used directly as a solid fuel source in the form of briquettes.
The Liquid Condensate: Bio-oil
As the hot gases produced during pyrolysis are cooled, a significant portion condenses into a dark, viscous liquid known as bio-oil or pyrolysis oil.
This liquid is a complex mixture of water, tars, and hundreds of organic compounds. While it has high energy density, it is not a direct substitute for conventional diesel or gasoline.
Its primary use is as a combustion fuel for industrial boilers and furnaces. With significant refining and upgrading, it can be converted into transportation fuels like biodiesel or serve as a source for specialty chemicals. A key advantage of bio-oil is its high energy density and stability, making it much easier to store and transport than gaseous fuels.
The Non-Condensable Gas: Syngas
Syngas is the portion of the output that remains a gas even after cooling. It is a mixture of combustible and non-combustible gases.
The typical composition includes hydrogen (H₂), carbon monoxide (CO), methane (CH₄), and carbon dioxide (CO₂).
In most modern pyrolysis plants, this gas is not wasted. It is immediately looped back into the system and burned to provide the heat required to sustain the pyrolysis reaction, dramatically improving the overall energy efficiency of the operation.
How Process Conditions Dictate the Output
The ratio of biochar, bio-oil, and syngas is not fixed. It is a direct result of the process parameters you choose, giving you significant control over the final output.
The Impact of Temperature and Speed
The rate and temperature of heating are the most critical levers you can pull.
Slow pyrolysis, which involves lower temperatures (around 400°C) and longer processing times, maximizes the yield of the solid byproduct, biochar.
Fast pyrolysis, by contrast, uses higher temperatures (above 500°C) and extremely rapid heating and cooling times (seconds). This process is specifically designed to maximize the yield of the liquid byproduct, bio-oil.
The Role of Feedstock
The initial material, or feedstock, fundamentally determines the chemical properties of the byproducts. Pyrolysis of woody biomass will produce a bio-oil and biochar with specific properties, while the pyrolysis of waste plastics will yield a more hydrocarbon-rich oil that resembles crude oil. The feedstock's moisture content and physical size also play a crucial role in process efficiency.
Understanding the Trade-offs and Realities
While versatile, pyrolysis and its byproducts come with practical limitations that must be understood for successful implementation.
Bio-oil is Not Crude Oil
It is crucial to recognize that raw bio-oil is highly acidic, corrosive to standard pipes and engines, and can be unstable over time. Using it as a fuel requires either specialized equipment designed to handle it or an expensive upgrading process to stabilize it and remove oxygen.
Biochar Quality Varies
The value of biochar is highly dependent on the feedstock and process conditions. Biochar intended for agricultural use must be free of contaminants, which may not be the case if mixed waste is used as a feedstock. Not all char is created equal.
The Economic Equation
The economic viability of a pyrolysis plant hinges on a delicate balance. It depends on securing a low-cost, consistent feedstock, the operational efficiency of the plant (especially the use of syngas for self-heating), and strong local markets for the specific byproducts being produced.
Making the Right Choice for Your Goal
Your strategy for implementing pyrolysis should be dictated by your primary objective.
- If your primary focus is carbon sequestration or soil improvement: You should optimize for slow pyrolysis to maximize the production of high-quality, stable biochar.
- If your primary focus is creating a transportable liquid fuel or chemical feedstock: You should optimize for fast pyrolysis to maximize the yield of bio-oil, with the understanding that it will likely require further refining.
- If your primary focus is on-site energy generation or maximum waste reduction: A balanced process that uses the syngas for heat and produces both biochar and bio-oil as valuable co-products is the most energy-efficient model.
By understanding these outputs and the levers that control them, you can engineer a pyrolysis process that effectively solves your specific economic or environmental challenge.
Summary Table:
| Byproduct | Physical State | Primary Characteristics | Common Uses |
|---|---|---|---|
| Biochar | Solid | Carbon-rich, stable residue | Soil amendment, carbon sequestration, solid fuel |
| Bio-Oil | Liquid | Complex, viscous liquid mixture | Industrial boiler fuel, feedstock for refined biofuels |
| Syngas | Gas | Mixture of combustible gases (H₂, CO, CH₄) | On-site heat source to sustain the pyrolysis process |
Ready to engineer a pyrolysis process for your specific needs? KINTEK specializes in high-quality laboratory equipment for analyzing and optimizing pyrolysis reactions. Whether you're researching biochar for agriculture, bio-oil for fuel, or syngas for energy, our tools provide the precision and reliability you need. Let our experts help you select the right equipment to achieve your goals—contact us today to discuss your project!
Visual Guide
Related Products
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Graphite Vacuum Continuous Graphitization Furnace
- Vertical Laboratory Tube Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Vertical High Temperature Graphite Vacuum Graphitization Furnace
People Also Ask
- Why is a quartz tube furnace utilized in the thermal oxidation of MnCr2O4 coatings? Unlock Precise Selective Oxidation
- How to clean a tube furnace? A Step-by-Step Guide for Safe and Effective Maintenance
- What precautions should be taken when using a tube furnace? Ensure Safe, Effective High-Temperature Processing
- What factors influence the general design of a tube furnace? Match Your Process with the Perfect System
- How does a quartz tube vacuum furnace contribute to the crystallization process of Ag-doped Li-argyrodite electrolytes?



















