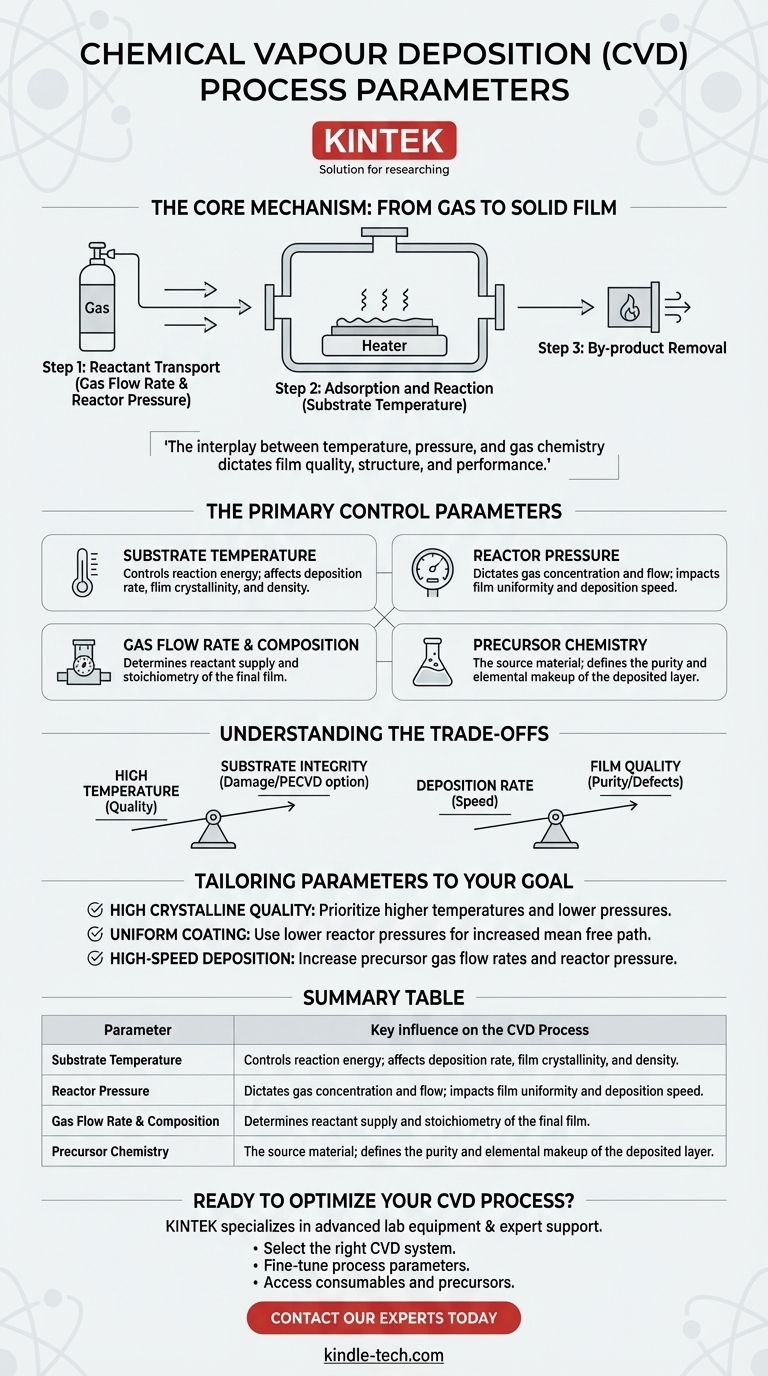
The key parameters in Chemical Vapor Deposition (CVD) are the interdependent variables that control the entire process, from the transport of gases to the final chemical reactions on a substrate. The most critical of these are substrate temperature, reactor pressure, gas flow rates and composition, and the specific precursor chemicals used. These parameters are not independent; adjusting one will invariably affect the others, influencing the final film's quality, thickness, and structure.
Understanding CVD parameters is not about memorizing a list, but about mastering a set of interconnected controls. The interplay between temperature, pressure, and gas chemistry is what ultimately dictates the quality, structure, and performance of the final deposited film.

The Core Mechanism: From Gas to Solid Film
To understand how parameters work, you must first understand the fundamental steps of the CVD process. It is a carefully orchestrated sequence where gaseous chemicals are transformed into a solid layer on a surface.
Step 1: Reactant Transport
The process begins by introducing reactant gases, known as precursors, into a reaction chamber. The gas flow rate and reactor pressure determine how these precursors travel to the substrate.
Step 2: Adsorption and Reaction
Once the precursor gases reach the substrate, they adsorb onto its surface. The substrate temperature provides the necessary energy for these precursors to decompose or react with other gases.
This surface reaction is the heart of CVD. It breaks down the precursor molecules, leaving behind the desired elements as a solid film and creating volatile by-products.
Step 3: By-product Removal
The gaseous by-products created during the reaction must be efficiently removed from the substrate surface and pumped out of the chamber. This final step is crucial for creating a pure, uncontaminated film.
The Primary Control Parameters Explained
Each parameter is a lever you can pull to influence the outcome of the deposition. Mastering these is key to achieving consistent and high-quality results.
Substrate Temperature
Temperature is arguably the most critical parameter. It directly provides the thermal energy required to drive the surface reactions.
A higher temperature generally increases the deposition rate and can improve the crystallinity and density of the film. However, excessively high temperatures can lead to unwanted gas-phase reactions or damage to the substrate itself.
Reactor Pressure
The pressure inside the chamber dictates the concentration of precursor molecules and the mean free path—the average distance a gas molecule travels before colliding with another.
Lower pressures (vacuum conditions) increase the mean free path, which can improve film uniformity, especially on complex, non-flat surfaces. Higher pressures increase reactant concentration near the surface, often leading to a faster deposition rate but potentially lower film quality.
Gas Flow Rate & Composition
The rate at which precursor gases are introduced controls the supply of reactant material to the substrate. The ratio of different gases is also critical.
Higher flow rates can increase the deposition rate up to a point, but if too high, they can lead to inefficient reactions as precursors are swept away before they can react. The chemical composition of the gas mixture determines the stoichiometry and elemental makeup of the final film.
Precursor Chemistry
The choice of precursor chemicals is a foundational parameter. These molecules are the source material for the film.
An ideal precursor is volatile enough to be transported as a gas but decomposes cleanly at the desired temperature, leaving behind a high-purity film and non-reactive by-products.
Understanding the Trade-offs
Optimizing a CVD process is always a matter of balancing competing factors. There is rarely a single "best" setting, only the best setting for a specific goal.
High Temperature vs. Substrate Integrity
One of the primary limitations of traditional CVD is its high operating temperature (often 850-1100°C). This provides the energy for high-quality, crystalline films.
However, many advanced materials and electronic components cannot withstand such heat. This trade-off has led to the development of plasma-enhanced CVD (PECVD), which uses a plasma to energize the gas, allowing for deposition at much lower temperatures.
Deposition Rate vs. Film Quality
There is often an inverse relationship between how fast you grow a film and its final quality. Pushing for a higher deposition rate by increasing temperature, pressure, or flow rate can introduce defects, impurities, and poor crystal structure into the film.
Conversely, achieving a highly pure, dense, and well-ordered crystalline film often requires slower, more controlled growth conditions, which reduces throughput.
Tailoring Parameters to Your Goal
The optimal parameters depend entirely on your desired outcome. Use these principles as a starting guide.
- If your primary focus is high crystalline quality: Prioritize higher substrate temperatures and lower pressures to allow for slow, ordered growth with minimal gas-phase contamination.
- If your primary focus is coating a complex shape uniformly: Use lower reactor pressures to increase the mean free path of the gas molecules, ensuring they can reach all surfaces.
- If your primary focus is high-speed deposition (throughput): Increase the precursor gas flow rates and reactor pressure to maximize the amount of reactant reaching the substrate surface, accepting a potential trade-off in film perfection.
Ultimately, mastering CVD is a process of balancing these parameters to precisely engineer the material properties your application demands.
Summary Table:
| Parameter | Key Influence on the CVD Process |
|---|---|
| Substrate Temperature | Controls reaction energy; affects deposition rate, film crystallinity, and density. |
| Reactor Pressure | Dictates gas concentration and flow; impacts film uniformity and deposition speed. |
| Gas Flow Rate & Composition | Determines reactant supply and stoichiometry of the final film. |
| Precursor Chemistry | The source material; defines the purity and elemental makeup of the deposited layer. |
Ready to Optimize Your CVD Process?
Achieving the perfect balance of temperature, pressure, and gas chemistry is key to producing high-quality thin films. KINTEK specializes in providing the advanced lab equipment and expert support you need to master your Chemical Vapor Deposition applications.
We help you:
- Select the right CVD system for your specific materials and goals.
- Fine-tune process parameters for optimal film quality and throughput.
- Access the consumables and precursors required for consistent, high-purity results.
Contact our experts today to discuss how we can enhance your laboratory's capabilities and drive your research forward.
Visual Guide
Related Products
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
People Also Ask
- What is the difference between PECVD and CVD? Unlock the Right Thin-Film Deposition Method
- What is PECVD in semiconductor? Enable Low-Temperature Thin Film Deposition for ICs
- What are the steps of the CVD process? A Guide to Precision Thin Film Deposition
- What color diamonds are CVD? Understanding the Process from Brown Tint to Colorless Beauty
- What is the vapor phase deposition technique? A Guide to PVD & CVD Thin-Film Coating Methods



















