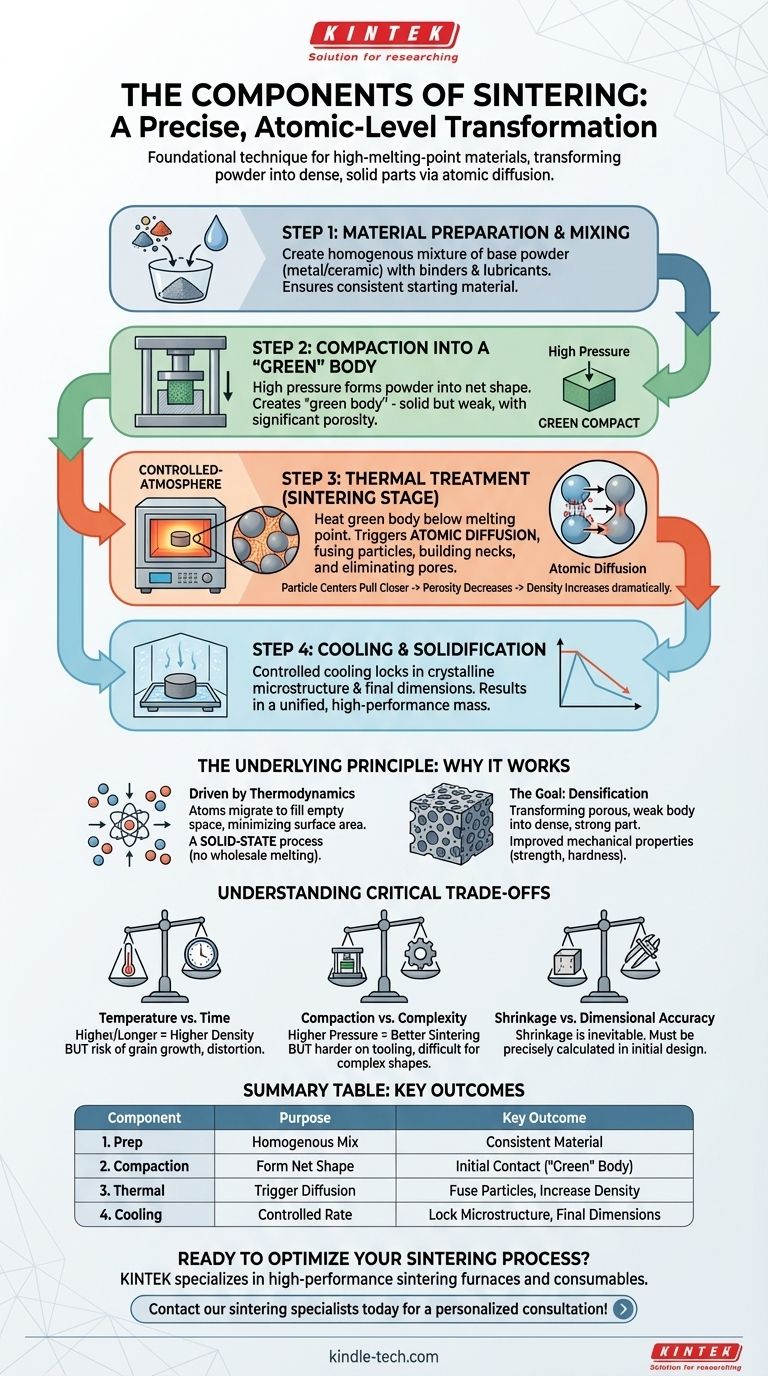
At its core, the sintering process consists of three primary components: preparing and compacting a powder material, applying targeted heat below its melting point, and controlled cooling. This thermal process triggers atomic diffusion between the powder particles, fusing them into a solid, dense mass without ever reaching a liquid state. It is a foundational technique in powder metallurgy and ceramics, especially for materials with extremely high melting points.
Sintering is not simply baking a powder; it is a precise, atomic-level transformation. Understanding its components moves you from seeing it as a recipe to mastering a powerful method for controlling a material's final density, strength, and performance.

The Foundational Components of the Sintering Process
While the specifics vary by material and desired outcome, the sintering workflow is universally built on a few key stages. Each step serves a distinct purpose in transforming loose powder into a unified, high-performance part.
Step 1: Material Preparation and Mixing
Before any heating occurs, the raw material must be prepared. This involves creating a homogenous mixture of the primary metal or ceramic powder.
Often, other substances are added. Binders act as a temporary glue to hold the particles together, while lubricants reduce friction during the pressing stage. Alloying elements, like copper powder, may also be introduced to enhance the final properties.
Step 2: Compaction into a 'Green' Body
The prepared powder is placed into a mold or die and subjected to high pressure. This step, known as compaction, forms the powder into the desired net shape.
The resulting object is called a "green" compact or green body. It is solid enough to be handled but is mechanically weak and brittle, with significant porosity between the particles. The primary goal of compaction is to create as much particle-to-particle contact as possible.
Step 3: Thermal Treatment (The Sintering Stage)
This is the heart of the process. The green body is placed in a controlled-atmosphere furnace and heated according to a precise thermal profile.
First, at lower temperatures, any residual lubricants or organic binders are burned off. As the temperature rises—approaching, but not reaching, the material's melting point—atomic diffusion begins. Atoms migrate across the boundaries where particles touch, effectively building "necks" or bridges between them.
These necks grow, pulling the particle centers closer together. This action systematically eliminates the empty pore spaces, causing the part to shrink and its density to increase dramatically.
Step 4: Cooling and Solidification
After holding at the sintering temperature for a set time, the component is cooled in a controlled manner.
This final stage is critical for locking in the desired crystalline microstructure and managing final dimensions. The part solidifies into a single, unified mass with properties far superior to the starting powder.
The Underlying Principle: Why Sintering Works
Understanding the steps is useful, but understanding the physics behind them is what allows for true process control. Sintering is fundamentally a battle against empty space within a material.
From Powder to Solid: The Role of Atomic Diffusion
Think of the powder particles as microscopic spheres. Compaction presses them together, but significant gaps remain. Heat acts as the catalyst, giving the atoms at the particle surfaces enough energy to move.
Driven by thermodynamics, these atoms migrate to fill the gaps between particles, minimizing surface area and creating a lower-energy state. This is a solid-state process; the material fuses together atom by atom, without the need for wholesale melting.
The Goal: Reducing Porosity and Increasing Density
The primary objective of sintering is to transform a porous, weak green body into a dense, strong final part.
As atomic diffusion closes the gaps between particles, the material's overall porosity decreases and its density increases. This densification is directly linked to improvements in mechanical properties like hardness, strength, and durability.
Understanding the Critical Trade-offs
Achieving a perfect sintered part requires balancing several competing factors. Mismanaging these trade-offs is the most common source of failure.
Temperature vs. Time
Higher sintering temperatures or longer hold times generally result in higher density. However, excessive heat can cause undesirable grain growth, which can make the material brittle. It also increases the risk of distortion or slumping.
Compaction vs. Complexity
Higher initial compaction pressure creates a denser green body with more particle contact, which can lead to better, more uniform sintering. However, extremely high pressure can be hard on tooling, and complex part geometries can make it difficult to achieve even density throughout the green compact.
Shrinkage vs. Dimensional Accuracy
Sintering is not a zero-change process; as porosity is eliminated, the part will shrink. This shrinkage can be significant and must be precisely calculated and accounted for in the initial mold design. Non-uniform shrinkage can lead to warping and a failure to meet dimensional tolerances.
Making the Right Choice for Your Goal
Understanding these components allows you to tailor the process to your specific objective.
- If your primary focus is maximum strength and density: Prioritize precise control over the thermal cycle and consider advanced methods like Liquid Phase Sintering (LPS), where a secondary material melts to accelerate densification.
- If your primary focus is producing complex geometries: Pay extra attention to the compaction stage to ensure uniform green density and meticulously calculate shrinkage to maintain dimensional accuracy.
- If your primary focus is fabricating high-melting-point materials: Recognize that sintering is often the only commercially viable path for materials like tungsten, molybdenum, or technical ceramics that are impractical to melt and cast.
By mastering these fundamental components, you can deliberately engineer the final properties of a material at the atomic level.
Summary Table:
| Component | Purpose | Key Outcome |
|---|---|---|
| 1. Powder Preparation | Create a homogenous mixture of base powder, binders, and lubricants. | Uniform starting material for consistent sintering. |
| 2. Compaction | Press powder into a 'green' body using high pressure in a mold. | Forms the net shape and creates initial particle contact. |
| 3. Thermal Treatment | Heat the green body below its melting point in a controlled furnace. | Triggers atomic diffusion, fusing particles and increasing density. |
| 4. Controlled Cooling | Cool the sintered part at a specific rate. | Locks in the final microstructure and dimensional accuracy. |
Ready to optimize your sintering process? The right lab equipment is critical for precise temperature control and consistent results. KINTEK specializes in high-performance sintering furnaces and consumables for powder metallurgy and ceramics. Our experts can help you select the perfect solution to achieve superior material density and strength.
Contact our sintering specialists today for a personalized consultation!
Visual Guide
Related Products
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1800℃ Muffle Oven Furnace for Laboratory
- Laboratory High Pressure Vacuum Tube Furnace
People Also Ask
- What is the technical value of using a quartz tube reaction chamber for static corrosion testing? Achieve Precision.
- What is a tubular furnace used for? Precision Heating for Material Synthesis & Analysis
- What materials are used for the tubes in tube furnaces? A Guide to Selecting the Right Tube for Your Process
- How do a quartz tube reactor and atmosphere furnace collaborate in Co@NC pyrolysis? Master Precision Synthesis
- How does a quartz tube vacuum furnace contribute to the crystallization process of Ag-doped Li-argyrodite electrolytes?



















