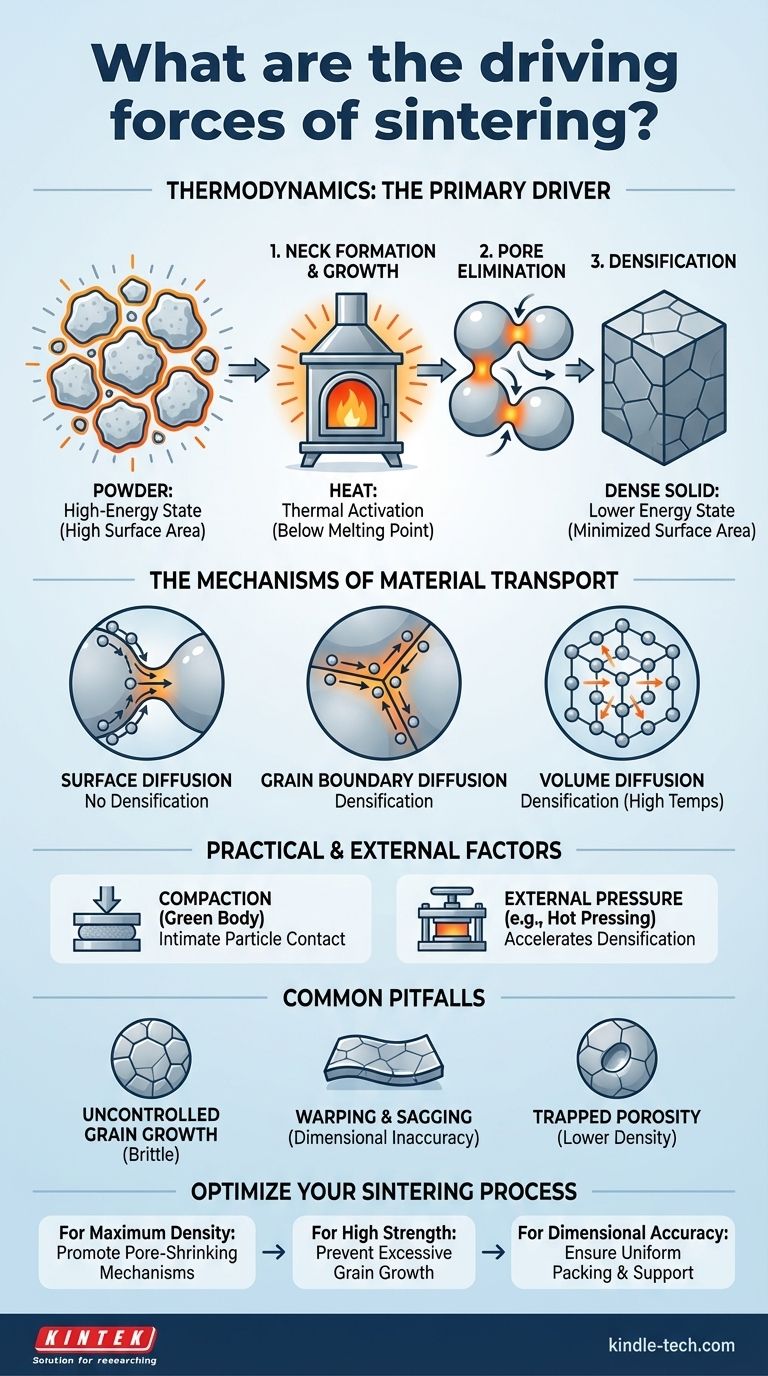
At its core, the primary driving force of sintering is thermodynamics. The process is driven by the significant reduction of surface free energy. A collection of fine powder particles possesses an enormous amount of surface area, which is an energetically unfavorable, high-energy state. By heating the material, you provide the atomic mobility necessary for the particles to bond, grow together, and reduce this total surface area, moving the system to a lower and more stable energy state.
Sintering is not simply about melting particles together. It is a thermally activated process where a material seeks to minimize its own internal energy by eliminating the high-energy surfaces between individual powder particles, resulting in a dense, solid mass.

The Fundamental Principle: Minimizing Surface Energy
Sintering is best understood as a material's natural tendency to reduce its energy. The process is governed by fundamental principles of physics and materials science, not just the application of heat.
Why Powder is a High-Energy State
A given mass of material has vastly more surface area as a fine powder than as a single solid block. The atoms on the surface of each particle are not fully bonded like the atoms in the interior, creating what is known as surface energy. This excess energy makes the powder system inherently unstable.
How Heat Unlocks the Process
The purpose of heating the material to a high temperature—yet below its melting point—is to provide energy. This energy doesn't melt the particles but instead gives their atoms enough kinetic energy to move. This atomic movement, or diffusion, is the mechanism by which the material can rearrange itself.
The Transformation to a Lower Energy State
Once the atoms can move, they begin to migrate to eliminate the high-energy surfaces. This occurs in stages:
- Neck Formation: Atoms diffuse to the points of contact between particles, forming small "necks" or bridges.
- Neck Growth: These necks grow larger, pulling the centers of the particles closer together.
- Pore Elimination: The spaces, or pores, between the particles gradually shrink and are eliminated as material is transported to fill the voids.
Each of these steps reduces the total surface area, thereby lowering the system's overall free energy and creating a denser, stronger component.
The Mechanisms of Material Transport
The reduction in surface energy is the "why," but atomic diffusion is the "how." Atoms move through several key pathways to reshape the material.
Surface Diffusion
Atoms migrate along the surface of the particles to the growing neck between them. This helps the necks form and grow but does not, by itself, cause the part to shrink or become denser.
Grain Boundary Diffusion
As necks form, they create a "grain boundary" between the original particles. Atoms can move rapidly along these boundaries, which is a highly effective mechanism for transporting material and shrinking pores, leading to densification.
Volume (Lattice) Diffusion
At the highest sintering temperatures, atoms can move directly through the crystal lattice of the particles themselves. This is often the dominant mechanism for the final stage of pore elimination and achieving maximum density.
Understanding the Practical Forces
While surface energy is the underlying driver, external factors are critical for initiating and controlling the process.
The Role of Compaction
Before heating, the powder is almost always pressed into a desired shape, known as a "green body." This initial compaction is crucial because it forces the particles into intimate contact, creating the starting points where diffusion and necking can begin.
The Role of External Pressure
In some advanced processes like Hot Pressing, pressure is applied during heating. This external pressure acts as an additional driving force, physically pushing the particles together and helping to collapse pores. It allows for densification at lower temperatures or in shorter times.
Common Pitfalls and Process Limitations
Controlling the driving forces of sintering is essential to avoid defects in the final part.
Uncontrolled Grain Growth
The same atomic diffusion that eliminates pores can also cause the grains within the material to grow excessively large. Overly large grains can often make the final material brittle and weak.
Warping and Sagging
During heating, before the part is fully dense and strong, it can be susceptible to gravity. If not supported correctly in the furnace, a part can warp or sag under its own weight, leading to dimensional inaccuracies.
Trapped Porosity
Sometimes, rapid grain growth can isolate pores within the center of a large grain. Once a pore is trapped like this, it is extremely difficult to remove, which limits the final density that can be achieved.
Making the Right Choice for Your Goal
By understanding the driving forces, you can manipulate the process parameters to achieve specific material properties.
- If your primary focus is maximum density: You must promote transport mechanisms that shrink pores, typically by using higher temperatures to activate volume diffusion and allowing sufficient time for pores to close.
- If your primary focus is high strength: You need to prevent excessive grain growth by using the lowest possible sintering temperature and time, or by adding specific chemical agents (dopants) that pin grain boundaries in place.
- If your primary focus is dimensional accuracy: You must ensure uniform powder packing during compaction and provide adequate support for the part in the furnace to prevent sagging and warping.
Understanding these fundamental drivers transforms sintering from a simple heating step into a powerful and precise tool for engineering advanced materials.
Summary Table:
| Driving Force | Mechanism | Effect |
|---|---|---|
| Reduction of Surface Free Energy | Atomic Diffusion (Surface, Grain Boundary, Volume) | Particles bond, necks form, and pores shrink |
| External Pressure (e.g., Hot Pressing) | Applied force during heating | Accelerates densification, lowers required temperature |
| Thermal Activation | Heating below melting point | Provides atomic mobility for material transport |
Ready to optimize your sintering process for superior material properties? At KINTEK, we specialize in advanced lab equipment and consumables tailored for materials science and laboratory needs. Whether you're aiming for maximum density, high strength, or precise dimensional accuracy, our expertise and solutions can help you achieve consistent, high-quality results. Contact our experts today to discuss how we can support your specific sintering challenges and enhance your research or production outcomes.
Visual Guide
Related Products
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1800℃ Muffle Oven Furnace for Laboratory
- Vacuum Heat Treat and Sintering Furnace with 9MPa Air Pressure
- Molybdenum Vacuum Heat Treat Furnace
People Also Ask
- How can you control temperature inside a resistance furnace? Master Precise Thermal Management
- What role do precision magnetic stirrers or homogenizers play in the synthesis of Cu-TiO2 sol-gel?
- What is gold sputtering for SEM? Prevent Charging and Enhance Image Quality for Non-Conductive Samples
- What are the equipment requirements for loading platinum (Pt) onto composite supports? Precise Stirring for High Dispersion
- What is the difference between batch furnace and continuous furnace? Choose the Right System for Your Production Volume
- What is the mechanism of RF reactive sputtering? Create High-Quality Insulating and Compound Films
- What is the importance of a laboratory electric constant temperature drying oven? Ensure Accurate Biomass Analysis
- How does hardness change with temperature? Understand the Inverse Relationship to Prevent Failure



















