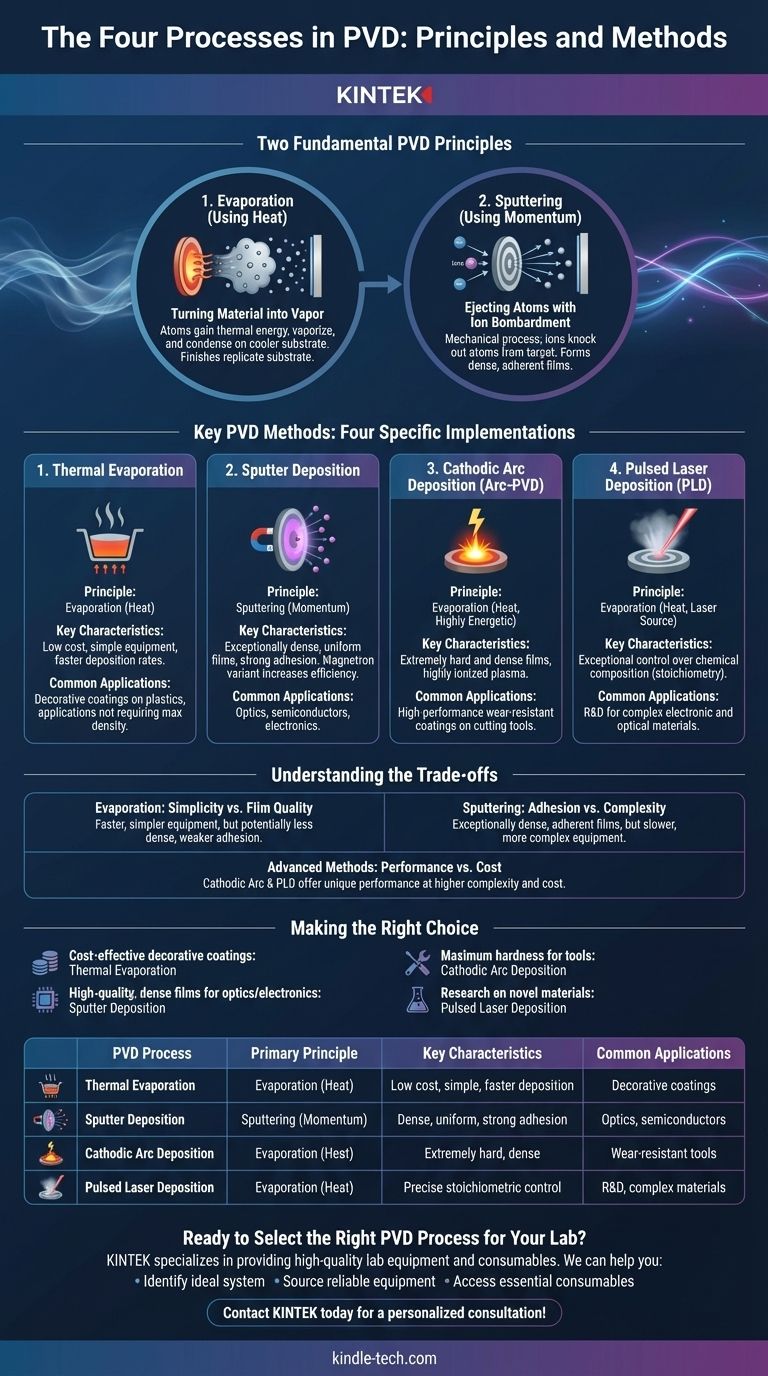
In practice, there are four commonly cited methods for Physical Vapor Deposition (PVD): Thermal Evaporation, Sputter Deposition, Cathodic Arc Deposition, and Pulsed Laser Deposition. These techniques represent the primary ways a solid material is converted into a vapor and then deposited as a thin film onto a substrate within a vacuum.
While we can name four distinct processes, the most effective way to understand PVD is to see it as a technology built on two fundamental principles: evaporation (using heat) and sputtering (using physical momentum). The specific methods are simply different ways of achieving one of these two outcomes.

The Two Fundamental PVD Principles
To truly grasp PVD, it's essential to look past a simple list of names and understand the core physics at play. Nearly all PVD processes are a variation of one of two mechanisms.
Evaporation: Turning Material into Vapor with Heat
This is the most intuitive principle. The source material, or "target," is heated in a vacuum until its atoms gain enough thermal energy to vaporize. This vapor then travels through the vacuum chamber and condenses onto the cooler substrate, forming a solid thin film.
The finish of the final product directly replicates the finish of the substrate it is coated on.
Sputtering: Ejecting Atoms with Ion Bombardment
Sputtering is a mechanical, not thermal, process. It begins by creating a plasma, typically from an inert gas like Argon. High-voltage fields accelerate the gas ions, causing them to collide with the target material with immense force.
These collisions physically knock out, or "sputter," atoms from the target. These ejected atoms then travel and deposit onto the substrate, creating a dense and highly adherent film.
Key PVD Methods Explained
The four processes you asked about are specific implementations of the principles above. Each offers a unique profile of speed, cost, and resulting film quality.
1. Thermal Evaporation
This is the simplest form of evaporative PVD. The source material is placed in a crucible and heated by a resistive element until it vaporizes.
It is a relatively low-cost and straightforward method, often used for coatings that do not require maximum density or adhesion, such as decorative finishes on plastics.
2. Sputter Deposition
This is the classic implementation of the sputtering principle. It is prized for creating films that are exceptionally dense, uniform, and have strong adhesion to the substrate.
Magnetron Sputtering is the most common variant, using powerful magnets behind the target to trap electrons. This dramatically increases the efficiency of the ionization process, leading to higher deposition rates.
3. Cathodic Arc Deposition (Arc-PVD)
Cathodic Arc is a highly energetic form of evaporation. It uses a high-current, low-voltage electric arc to create a small, intensely hot spot on the target's surface.
This spot instantly vaporizes the material and creates a highly ionized plasma. The resulting films are extremely hard and dense, making this method ideal for high-performance wear-resistant coatings on cutting tools.
4. Pulsed Laser Deposition (PLD)
PLD is another evaporative technique that uses a high-power pulsed laser as its energy source. The laser beam is focused onto the target, ablating the material and creating a plume of vapor that deposits onto the substrate.
This method offers exceptional control over the film's chemical composition (stoichiometry), making it a powerful tool for research and development of complex electronic and optical materials.
Understanding the Trade-offs
No single PVD process is universally superior; the choice is always a matter of balancing competing priorities.
Evaporation: Simplicity vs. Film Quality
Evaporation methods like thermal and e-beam are generally faster and use simpler equipment. However, the lower energy of the vaporized atoms can result in films that are less dense and have weaker adhesion compared to sputtered films.
Sputtering: Adhesion vs. Complexity
Sputtering produces exceptionally dense and adherent films, making it ideal for high-performance applications like semiconductors. The trade-off is typically a slower deposition rate and more complex, expensive equipment.
Advanced Methods: Performance vs. Cost
Processes like Cathodic Arc and PLD offer unique performance benefits—extreme hardness for Arc-PVD and compositional control for PLD. This performance comes at the cost of higher equipment complexity, operational challenges, and overall expense.
Making the Right Choice for Your Application
Your specific goal determines which process is the most logical choice.
- If your primary focus is cost-effective decorative coatings: Thermal Evaporation provides a simple and efficient solution.
- If your primary focus is high-quality, dense films for optics or electronics: Sputter Deposition, especially Magnetron Sputtering, offers superior film quality and uniformity.
- If your primary focus is maximum hardness and wear resistance for tools: Cathodic Arc Deposition creates the robust films required for demanding mechanical applications.
- If your primary focus is research on novel materials with complex chemistry: Pulsed Laser Deposition provides the precision needed to control film stoichiometry.
Understanding these core principles and trade-offs empowers you to select the right tool for the job.
Summary Table:
| PVD Process | Primary Principle | Key Characteristics | Common Applications |
|---|---|---|---|
| Thermal Evaporation | Evaporation (Heat) | Low cost, simple, faster deposition | Decorative coatings on plastics |
| Sputter Deposition | Sputtering (Momentum) | Dense, uniform films, strong adhesion | Optics, semiconductors, electronics |
| Cathodic Arc Deposition | Evaporation (Heat) | Extremely hard, dense, highly ionized plasma | Wear-resistant coatings on cutting tools |
| Pulsed Laser Deposition | Evaporation (Heat) | Precise stoichiometric control, complex materials | R&D for novel electronic/optical materials |
Ready to Select the Right PVD Process for Your Lab?
Choosing the optimal PVD method is critical for achieving your desired coating results, whether for research, development, or production. KINTEK specializes in providing high-quality lab equipment and consumables tailored to your specific PVD needs.
We can help you:
- Identify the ideal PVD system based on your application requirements for film quality, adhesion, and cost.
- Source reliable equipment for Thermal Evaporation, Sputtering, Cathodic Arc, or Pulsed Laser Deposition processes.
- Access essential consumables to ensure consistent and high-performance thin film deposition.
Don't leave your coating results to chance. Let our expertise guide you to the perfect PVD solution for your laboratory's unique challenges.
Contact KINTEK today for a personalized consultation and enhance your thin film capabilities!
Visual Guide
Related Products
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- Electron Beam Evaporation Coating Oxygen-Free Copper Crucible and Evaporation Boat
- VHP Sterilization Equipment Hydrogen Peroxide H2O2 Space Sterilizer
People Also Ask
- What are the applications of PECVD? Essential for Semiconductors, MEMS, and Solar Cells
- How are PECVD and CVD different? A Guide to Choosing the Right Thin-Film Deposition Process
- Why does PECVD commonly use RF power input? For Precise Low-Temperature Thin Film Deposition
- What is the principle of plasma enhanced chemical vapor deposition? Achieve Low-Temperature Thin Film Deposition
- What are the advantages of PECVD? Enable Low-Temperature, High-Quality Thin-Film Deposition



















