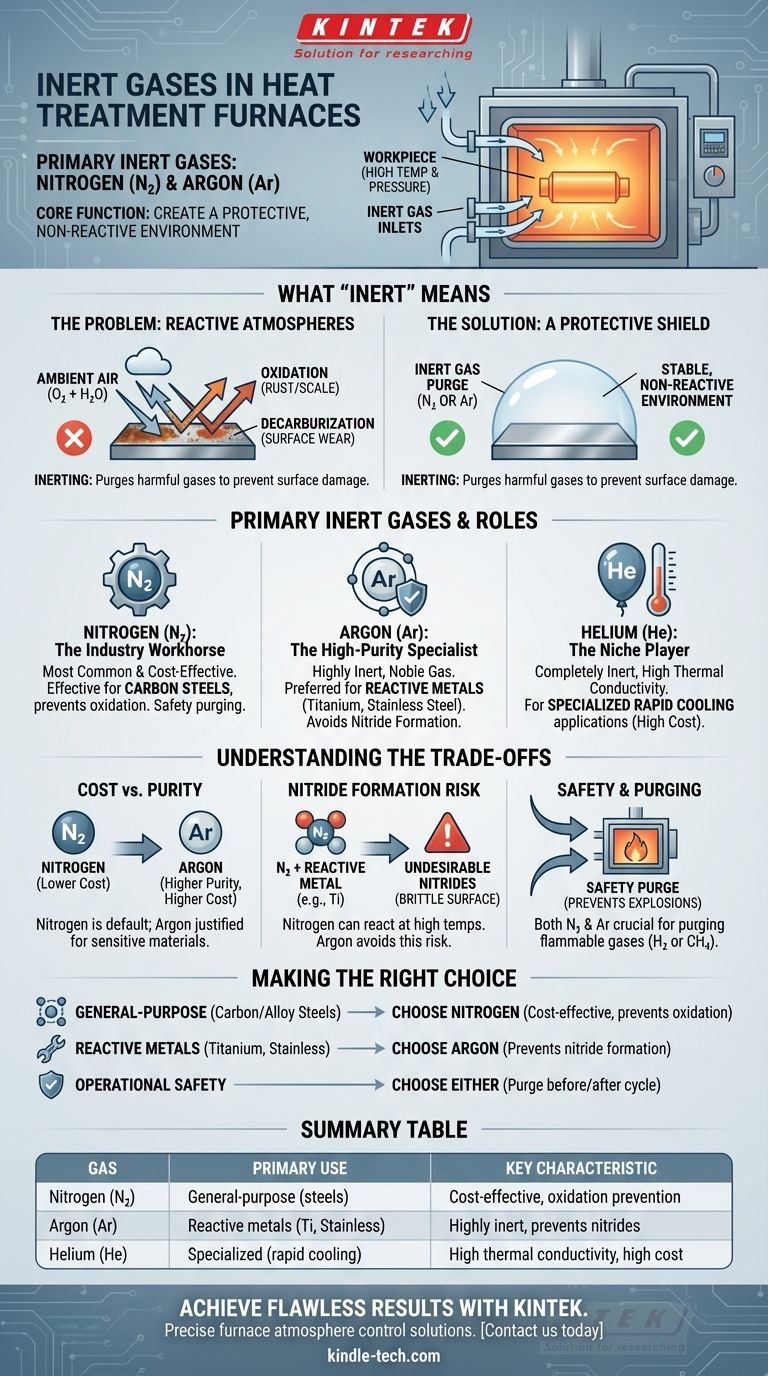
In heat treatment, the primary inert gases used are nitrogen and argon. These gases are chosen because they do not readily react with the metal parts being treated, even at high temperatures. They are introduced into a furnace to displace reactive gases like oxygen, which can cause undesirable effects like oxidation and decarburization that degrade the final quality of the component.
The core function of an inert gas in a heat treatment furnace is not simply to be present, but to actively create a protective, non-reactive environment. This controlled atmosphere is essential for preventing damage to the workpiece and ensuring the safe operation of the furnace.

What "Inert" Means in Heat Treatment
In this specific context, the term "inert" goes beyond its basic chemical definition. It refers to a gas that will not chemically react with the metal workpiece under the specific conditions of high temperature and pressure inside a furnace.
The Problem: Reactive Atmospheres
If a furnace is filled with ambient air, the oxygen and water vapor present will react aggressively with the hot metal surface.
This reaction leads to two primary problems: oxidation (scaling or rust) and decarburization (loss of carbon from the surface of steel), both of which severely compromise the material's strength and integrity.
The Solution: A Protective Shield
Introducing an inert gas purges the furnace, pushing out these harmful reactive gases.
This process, known as inerting, envelops the workpiece in a stable, protective atmosphere, ensuring the heat treatment process modifies the metal's properties as intended without causing surface damage.
The Primary Inert Gases and Their Roles
While several gases are used in furnace atmospheres, only a select few are truly inert. The choice between them typically comes down to the specific metal being treated and the cost of the gas.
Nitrogen (N₂): The Industry Workhorse
Nitrogen is the most common and cost-effective inert gas used for heat treatment.
It is highly effective at displacing oxygen and preventing oxidation for a wide range of common materials, like carbon steels. It is also used extensively for safety purging of flammable atmospheres during startup and shutdown.
Argon (Ar): The High-Purity Specialist
Argon, a noble gas, is more chemically inert than nitrogen.
It is the preferred choice when treating highly reactive metals, such as titanium, certain stainless steels, or refractory metals. In these cases, even nitrogen can react at high temperatures to form undesirable nitrides on the metal's surface, a problem that argon completely avoids.
Helium (He): The Niche Player
Helium is another noble gas that is completely inert.
However, its high cost limits its use to very specific applications where its unique properties, like high thermal conductivity for rapid cooling, are required.
Understanding the Trade-offs
Selecting the right inert gas is a balance of process requirements, material compatibility, and budget.
Cost vs. Purity
Nitrogen is significantly less expensive than argon, making it the default choice for most applications.
The added cost of argon is only justified when the material being treated is sensitive enough to react with nitrogen.
The Risk of Nitride Formation
The critical trade-off is nitrogen's potential reactivity. While inert for most steels, it can form nitrides on the surface of metals like titanium or some high-chromium steels.
This can make the surface brittle and is often undesirable. If nitride formation is a risk for your specific alloy, argon is the only safe choice.
Safety and Purging
Both nitrogen and argon are crucial for safety. They are used to purge the furnace of flammable process gases (like hydrogen or methane) before opening the doors, preventing explosions when the hot atmosphere mixes with air.
Making the Right Choice for Your Process
Your decision should be guided by the material you are treating and your operational goals.
- If your primary focus is general-purpose treatment of carbon and alloy steels: Nitrogen is the most practical and cost-effective choice for preventing oxidation.
- If your primary focus is treating reactive metals like titanium or certain stainless steels: Argon is required to prevent the formation of unwanted nitrides on the material's surface.
- If your primary focus is operational safety: Either nitrogen or argon should be used to purge flammable or reactive gases from the furnace before and after a treatment cycle.
Ultimately, mastering your furnace atmosphere by choosing the correct inert gas is fundamental to achieving consistent, high-quality results in heat treatment.
Summary Table:
| Inert Gas | Primary Use | Key Characteristic |
|---|---|---|
| Nitrogen (N₂) | General-purpose for carbon/alloy steels | Cost-effective, excellent for oxidation prevention |
| Argon (Ar) | Reactive metals (e.g., titanium, stainless steels) | Highly inert, prevents nitride formation |
| Helium (He) | Specialized applications requiring rapid cooling | High thermal conductivity, high cost |
Achieve flawless heat treatment results with the right inert gas atmosphere. KINTEK specializes in lab equipment and consumables, providing solutions for precise furnace atmosphere control. Our experts can help you select the optimal gas for your specific metals and processes, ensuring superior quality and safety. Contact us today to optimize your heat treatment operations!
Visual Guide
Related Products
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
- Vertical Laboratory Tube Furnace
People Also Ask
- Why nitrogen is used in furnace? A Cost-Effective Shield for High-Temperature Processes
- Can nitrogen gas be heated? Leverage Inert Heat for Precision and Safety
- Why nitrogen is used in annealing furnace? To prevent oxidation and decarburization for superior metal quality
- How do you make an inert atmosphere? Master Safe, Pure Processes with Inerting
- What is an inert atmosphere heat treatment? Protect Your Metals from Oxidation & Decarburization



















