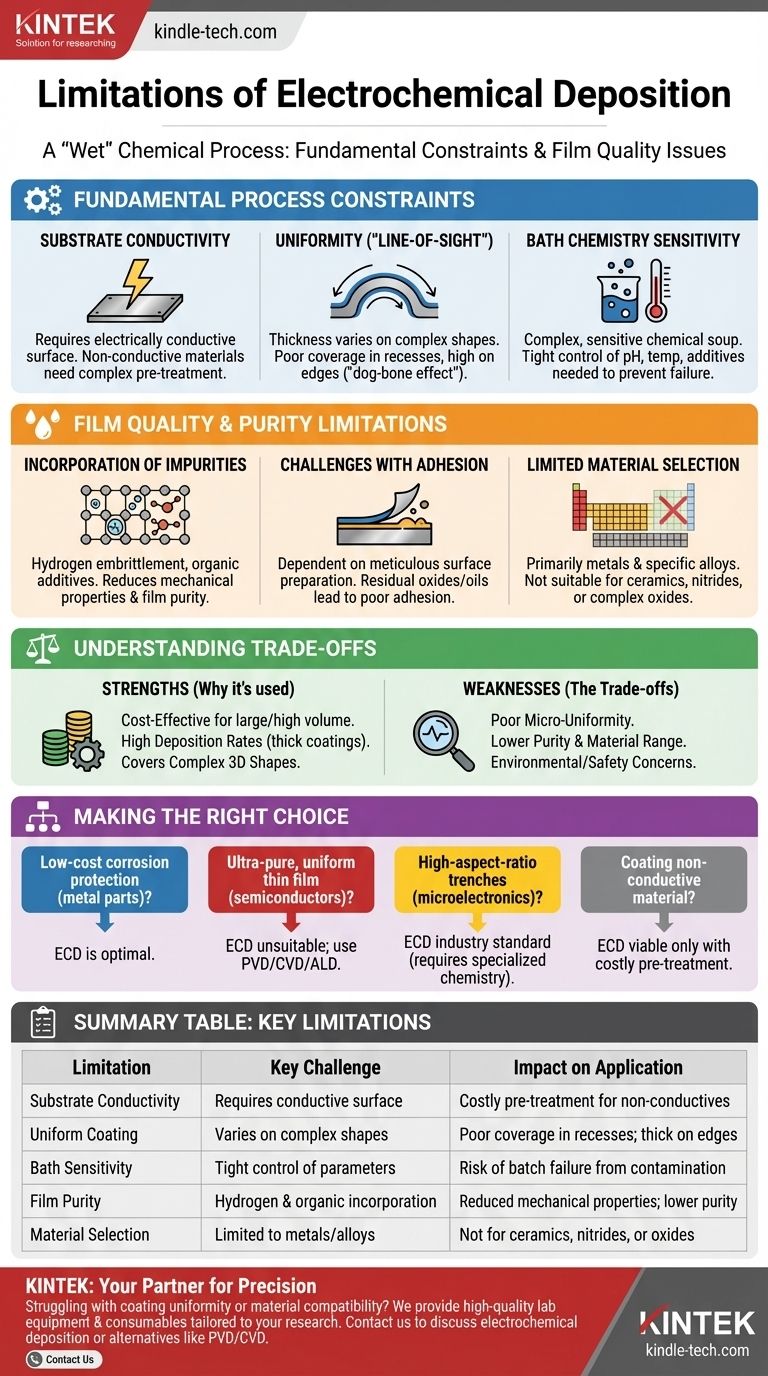
The primary limitations of electrochemical deposition are its requirement for a conductive substrate, challenges in achieving uniform coating thickness on complex shapes, sensitivity to bath chemistry, and potential for film contamination from the electrolyte solution. These factors constrain its use in applications demanding high purity or precise nanoscale uniformity.
Electrochemical Deposition (ECD) is a powerful and cost-effective method for creating metal coatings, but it is fundamentally a "wet" chemical process governed by electrochemical principles. Its limitations arise directly from this nature, contrasting sharply with "dry" vacuum-based methods like PVD or CVD.

Fundamental Process Constraints
The core mechanics of using an electrical current in a liquid chemical bath give rise to several inherent limitations that must be understood before selecting this process.
Substrate Conductivity Requirement
The workpiece you intend to coat must be electrically conductive. The substrate acts as the cathode in the electrochemical cell, and if it cannot carry a current, the deposition process will not occur.
While non-conductive materials like plastics can be coated, they first require a complex and often costly pre-treatment process to make their surface conductive, such as through electroless plating.
The "Line-of-Sight" Problem
Deposition is not uniform across a geometrically complex surface. The electric field and current density are naturally higher on protruding features and sharp corners ("high-current-density areas") and lower in recesses or holes ("low-current-density areas").
This leads to a thicker coating on external corners and a much thinner—or even non-existent—coating in deep recesses. This phenomenon, sometimes called the "dog-bone effect" in trench-filling applications, is a major challenge for creating highly uniform films.
Complex and Sensitive Bath Chemistry
The electrolyte bath is a complex chemical soup that requires extremely tight process control. The final film properties are highly sensitive to small variations in its composition.
Key parameters like pH, temperature, ion concentration, and the presence of additives must be constantly monitored and maintained. Contamination of the bath can quickly ruin an entire batch, and the formulation of additives is often a proprietary art.
Environmental and Safety Concerns
The chemical baths used in ECD often contain hazardous materials. This can include heavy metals, strong acids, or highly toxic compounds like cyanide (used in some gold or copper plating baths).
Managing, treating, and disposing of this chemical waste is a significant environmental and cost consideration. It also necessitates stringent safety protocols to protect workers.
Limitations in Film Quality and Purity
Beyond the operational constraints, the nature of the process also places limits on the quality of the final deposited film.
Incorporation of Impurities
Unlike vacuum processes that occur in a highly controlled environment, ECD happens in a liquid solution. This creates opportunities for unwanted elements to co-deposit into the growing film.
The most common issue is hydrogen embrittlement, where hydrogen generated during the process gets trapped in the metal, making it brittle. Organic additives from the bath can also be incorporated, reducing the film's purity.
Challenges with Adhesion
Achieving strong adhesion between the deposited film and the substrate is not guaranteed. It is critically dependent on meticulous surface preparation.
Any residual oxides, oils, or other contaminants on the substrate surface will create a weak interface, leading to poor adhesion and potential flaking or peeling of the coating.
Limited Material Selection
Electrochemical deposition is primarily suited for metals and some specific alloys. While some metal oxides or conductive polymers can be deposited, the range of materials is far narrower than with other techniques.
Methods like Physical Vapor Deposition (PVD) or Chemical Vapor Deposition (CVD) can deposit a much wider array of materials, including ceramics, nitrides, and complex oxides.
Understanding the Trade-offs
Despite its limitations, ECD remains a vital industrial process because its weaknesses are balanced by significant strengths in specific contexts. The key is understanding the trade-offs.
Cost and Scalability
For coating large parts or high volumes of smaller parts, ECD is often significantly cheaper than vacuum-based alternatives. The equipment does not require expensive high-vacuum pumps, and the process is well-suited for batch production.
Deposition Rate and Thickness
ECD can achieve very high deposition rates, making it ideal for applying thick, protective coatings (hundreds of microns or more). This is often impractical or too time-consuming for methods like PVD or sputtering.
Coating of Complex 3D Shapes
While ECD struggles with uniform thickness on a microscopic scale (like in trenches), it excels at completely covering large, non-planar, or complex 3D objects. It can "throw" material around corners in a way that purely line-of-sight PVD processes cannot.
Making the Right Choice for Your Goal
To determine if ECD is the appropriate technology, you must evaluate its limitations against the primary requirements of your application.
- If your primary focus is low-cost corrosion protection on metal parts: ECD is almost certainly the optimal choice due to its cost-effectiveness and high deposition rate.
- If your primary focus is an ultra-pure, uniform thin film for semiconductors or optics: ECD is likely unsuitable; PVD, CVD, or Atomic Layer Deposition (ALD) offer far greater precision and purity.
- If your primary focus is filling high-aspect-ratio trenches in microelectronics (e.g., copper interconnects): ECD is the industry standard, but it requires highly specialized and complex additive chemistry to overcome its natural limitations.
- If your primary focus is coating a non-conductive material like plastic or ceramic: ECD is only viable if you can justify the additional cost and complexity of a surface metallization pre-treatment step.
Ultimately, choosing a deposition technology requires a clear understanding of not just what a process can do, but also what it cannot.
Summary Table:
| Limitation | Key Challenge | Impact on Application |
|---|---|---|
| Substrate Conductivity | Requires electrically conductive surface | Non-conductive materials need costly pre-treatment |
| Uniform Coating | Thickness varies on complex shapes | Poor coverage in recesses; thick on edges |
| Bath Sensitivity | Tight control of pH, temperature, additives | Risk of batch failure from contamination |
| Film Purity | Hydrogen embrittlement; organic incorporation | Reduced mechanical properties; lower purity |
| Material Selection | Primarily limited to metals/alloys | Not suitable for ceramics, nitrides, or oxides |
Struggling with coating uniformity or material compatibility? The right lab equipment is critical for selecting and optimizing your deposition process. At KINTEK, we specialize in providing high-quality lab equipment and consumables tailored to your research and production needs. Whether you're evaluating electrochemical deposition or advanced alternatives like PVD/CVD, our experts can help you identify the perfect solution for high-purity, uniform coatings. Contact us today via our [#ContactForm] to discuss how we can support your laboratory's success!
Visual Guide
Related Products
- Lab Electrochemical Workstation Potentiostat for Laboratory Use
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Electron Beam Evaporation Coating Oxygen-Free Copper Crucible and Evaporation Boat
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- Electrolytic Electrochemical Cell for Coating Evaluation
People Also Ask
- What is the function of a dual-electrode electrolytic cell in EPD? Enhance Ceramic Coating Precision
- What are the advantages of using electrochemical catalysis equipment for fuels from seawater? Streamlined Marine Energy
- What is the primary function of a three-electrode electrolytic cell? Isolate and Optimize PEC Device Performance
- What preparations are needed for the electrolyte before an experiment? A Guide to Flawless Electrochemical Results
- What is the proper procedure for post-experiment cleanup and storage of an all-quartz electrolytic cell? Ensure Longevity and Reproducibility
- How does thinning the radiation window of an in-situ cell improve imaging? Boost Clarity for Electrochemical Research
- What are the standard aperture specifications for the non-sealed and sealed electrolytic cells? Choose the Right Setup for Your Experiment
- What is the procedure for using a laboratory electrolytic etching device? Master 304L Stainless Steel Characterization



















