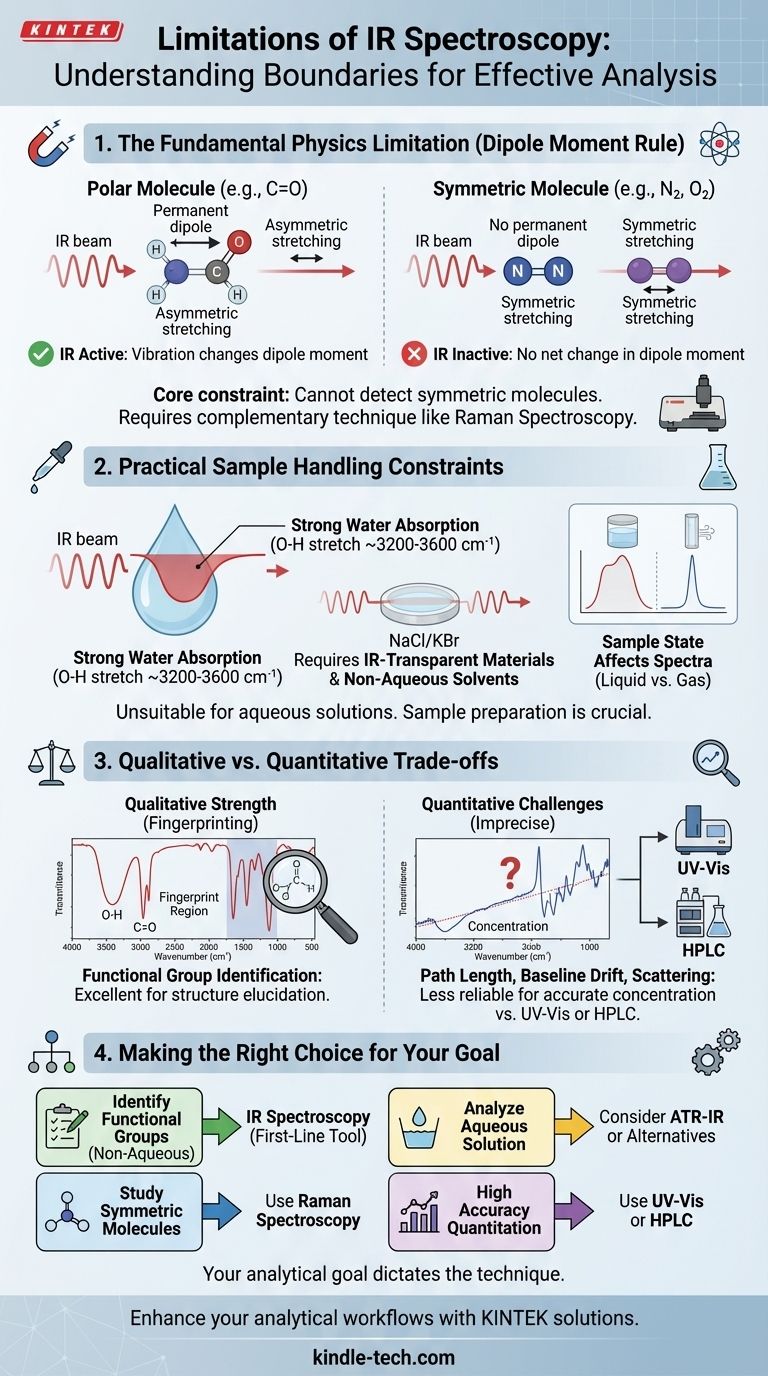
While incredibly powerful for identifying molecular structure, Infrared (IR) spectroscopy is not a universally applicable tool. Its primary limitations stem from a fundamental rule of physics: a molecule's vibration must cause a change in its dipole moment to be detected. Furthermore, practical challenges, particularly the strong IR absorption of water and the complexity of spectra from large molecules, define the boundaries of its effective use.
The core limitation of IR spectroscopy is its inability to detect vibrations from perfectly symmetric molecules. This, combined with its general unsuitability for analyzing aqueous solutions, means analysts must be deliberate in choosing when and how to apply this technique.

The Fundamental Limitation: The Dipole Moment Rule
The most significant constraint of IR spectroscopy is not instrumental but physical. For a molecule to absorb IR radiation, its vibration or rotation must cause a net change in the molecule's dipole moment.
What Makes a Vibration "IR Active"?
A bond with a dipole moment, like the carbonyl group (C=O), has a permanent separation of charge. As this bond stretches and compresses, the magnitude of that dipole moment changes, allowing it to absorb IR radiation at a characteristic frequency. This absorption event creates a peak in the IR spectrum.
When This Rule Fails: Symmetric Molecules
If a vibration does not cause a change in the dipole moment, it is "IR inactive" and will not produce a signal. This is most common in homonuclear diatomic molecules like oxygen (O₂) and nitrogen (N₂).
Similarly, perfectly symmetric molecules like carbon tetrachloride (CCl₄) may have individual polar bonds, but their symmetric vibrations cancel each other out, resulting in no net change in the dipole moment and thus weak or absent IR signals.
The Practical Implication: Complementary Techniques
Because of this limitation, IR spectroscopy cannot be used to study many simple, symmetric molecules. In these cases, analysts turn to a complementary method, Raman spectroscopy, which detects vibrations based on changes in polarizability, not dipole moment.
Practical Constraints in Sample Handling
Beyond the physics, the practical realities of sample preparation present major hurdles. The materials used must be compatible with the analysis, which is not always possible.
The Problem with Water
Water is a very poor solvent for IR analysis. It is a highly polar molecule with intense, broad absorption bands that can completely obscure the signals from the sample of interest, especially in the O-H stretching region (~3200-3600 cm⁻¹). This makes analyzing samples in aqueous solution exceptionally difficult.
The Need for IR-Transparent Materials
As a result, the sample holder and matrix must be transparent to IR radiation. Analysts commonly use polished salt plates made from sodium chloride (NaCl) or potassium bromide (KBr). This requires the sample to be either a neat liquid, a solid ground into a KBr pellet, or dissolved in a non-polar, IR-inactive solvent like carbon tetrachloride.
Sample State and Its Effect on Spectra
The physical state of a sample (solid, liquid, or gas) can significantly alter its IR spectrum. For example, the O-H stretch of an alcohol in a liquid state will be a broad peak due to hydrogen bonding, while the same alcohol in a dilute gaseous state will show a sharp, narrow peak. This variability requires careful control and consideration during interpretation.
Understanding the Trade-offs: Qualitative vs. Quantitative
IR spectroscopy is fundamentally a qualitative tool, and attempts to use it for quantitative measurements are often met with challenges.
IR's Strength: A Tool for Functional Group Identification
The primary power of IR is its ability to quickly and definitively identify the presence or absence of specific functional groups (e.g., C=O, O-H, N-H, C≡N). The spectrum acts as a molecular "fingerprint" that helps elucidate a compound's structure.
The Challenge of Quantitative Work
While Beer's Law can be applied to IR spectroscopy for quantitative analysis, it is often imprecise. The path length of the sample is difficult to control accurately, especially in solid KBr pellets. Furthermore, instrumental baseline drift and scattering effects can introduce significant errors, making techniques like UV-Vis or chromatography far more reliable for determining concentration.
Interpreting Complex Spectra
For large and complex molecules, the "fingerprint region" (below 1500 cm⁻¹) can become a dense and convoluted mess of overlapping peaks. While unique to the molecule, deciphering every single peak in this region is often impossible, making it difficult to distinguish between very similar isomers.
Making the Right Choice for Your Goal
Understanding these limitations is key to using IR spectroscopy effectively. Your analytical goal should dictate whether IR is the appropriate technique.
- If your primary focus is identifying functional groups in a non-aqueous organic compound: IR spectroscopy is an excellent, fast, and reliable first-line tool.
- If your primary focus is analyzing a sample in an aqueous solution: You must consider alternatives or specialized ATR-IR techniques to mitigate water's overwhelming interference.
- If your primary focus is studying symmetric molecules (like N₂ or S₈): You will need to use a complementary technique like Raman spectroscopy, as these molecules are IR inactive.
- If your primary focus is quantifying a component with high accuracy: You should prioritize a technique built for quantitation, such as UV-Vis spectroscopy or high-performance liquid chromatography (HPLC).
By recognizing its boundaries, you can leverage IR spectroscopy as the powerful structural elucidation tool it was designed to be.
Summary Table:
| Limitation Category | Key Constraint | Practical Implication |
|---|---|---|
| Fundamental Physics | Requires a change in dipole moment (IR-active vibration) | Cannot detect symmetric molecules (e.g., O₂, N₂); use Raman spectroscopy as a complement |
| Sample Handling | Strong IR absorption by water; requires IR-transparent materials (e.g., NaCl, KBr pellets) | Unsuitable for aqueous solutions; limits solvent and sample preparation options |
| Analytical Application | Primarily qualitative; challenging for quantitative measurements | Less reliable for concentration analysis compared to UV-Vis or HPLC; complex spectra hinder isomer differentiation |
Need precise analytical solutions for your laboratory? At KINTEK, we specialize in providing high-quality lab equipment and consumables tailored to your research needs. Whether you're working with IR spectroscopy or complementary techniques like Raman or HPLC, our products ensure accuracy and reliability. Contact us today to explore how our solutions can enhance your analytical workflows and overcome technical limitations!
Visual Guide
Related Products
- Iridium Dioxide IrO2 for Water Electrolysis
- Infrared High Resistance Single Crystal Silicon Lens
- Graphite Vacuum Furnace IGBT Experimental Graphitization Furnace
- Optical Window Glass Substrate Wafer Sheets Zinc Sulfide ZnS Window
- Lab Infrared Press Mold
People Also Ask
- What is the difference between oxidizing and reducing environments? Key Insights for Chemical Reactions
- What are the 3 types of electrode? A Guide to Anode, Cathode, Active, and Inert Electrodes
- What is bio-oil composed of? The Complex Chemistry of a Sustainable Fuel
- Which solvent is normally used in IR spectroscopy? Optimize Your Sample Prep for Clearer Results
- What are the elements of bio-oil? Unlocking the Chemistry of Renewable Fuel