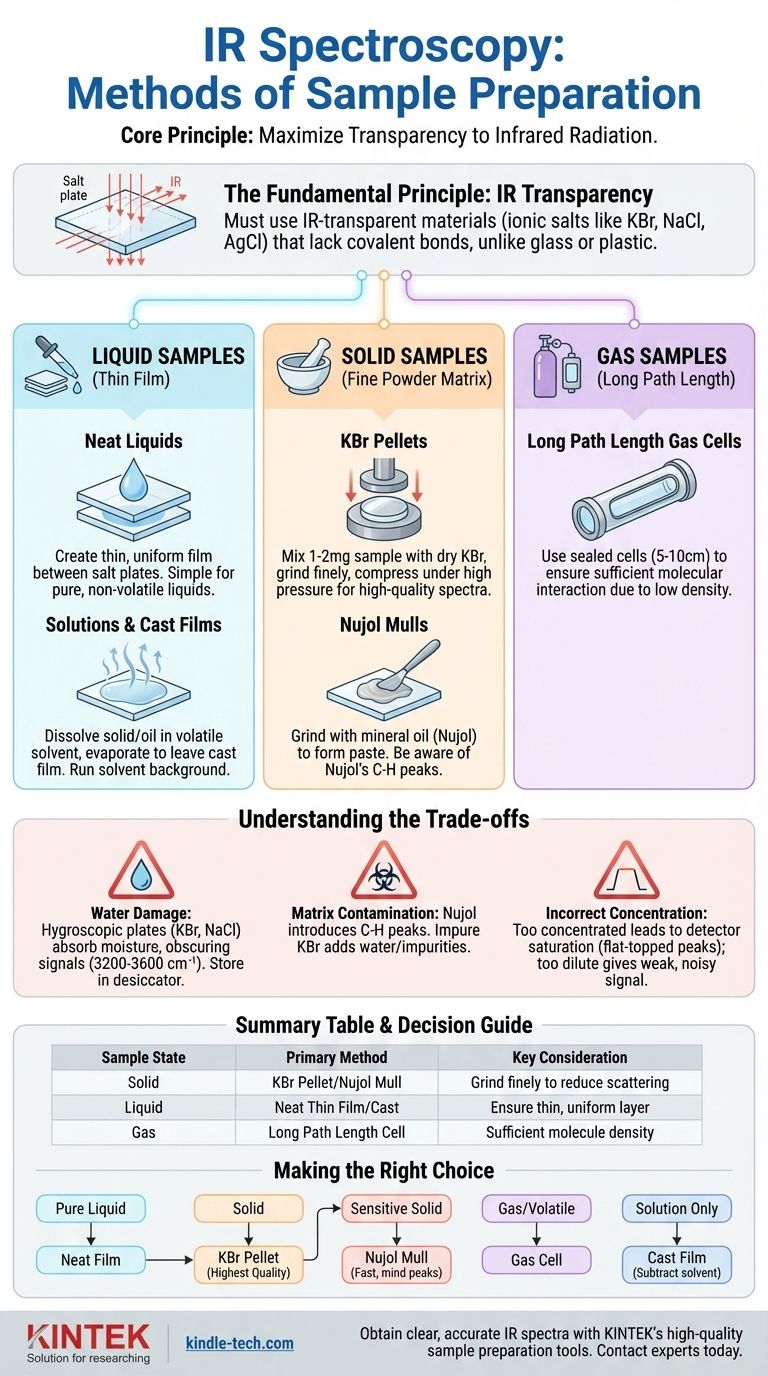
The primary methods for preparing a sample for Infrared (IR) spectroscopy depend on its physical state: solid, liquid, or gas. For liquids, a thin film is created between two salt plates. Solids are typically ground into a fine powder and either pressed into a transparent pellet with potassium bromide (KBr) or suspended in mineral oil (a Nujol mull). Gas samples are analyzed in a specialized cell with a long path length to ensure sufficient interaction with the IR beam.
The core principle of all IR sample preparation is to present the compound to the spectrometer in a form that is mostly transparent to infrared radiation. The chosen method must avoid introducing interfering signals while ensuring the sample's concentration is high enough for detection but not so high that it saturates the detector.

The Fundamental Principle: IR Transparency
The first and most critical rule in IR spectroscopy is that any material holding your sample must be transparent to infrared light. This is why standard glass or plastic cuvettes cannot be used, as their molecular bonds absorb strongly in the IR region, obscuring the signal from your sample.
Why Salt Plates are Used
To overcome this, sample holders are constructed from ionic salts. These materials lack covalent bonds that absorb in the mid-IR range, making them effectively invisible to the spectrometer.
Commonly used salts include Potassium Bromide (KBr) and Sodium Chloride (NaCl). These are relatively inexpensive and transparent over a wide range but are brittle and water-soluble. For aqueous solutions, a more robust material like Silver Chloride (AgCl) is required.
Preparing Liquid Samples
Liquid samples are often the simplest to prepare for IR analysis. The goal is to create a very thin layer for the IR beam to pass through.
Neat Liquids (Thin Film)
For pure, non-volatile liquids, this is the most direct method. A single drop of the liquid is placed on one salt plate, and a second plate is carefully placed on top.
The plates are gently pressed and rotated to spread the liquid into a thin, uniform film. The assembled plates are then placed in the spectrometer's sample holder.
Solutions and Cast Films
If your compound is a solid or a viscous oil, it can be dissolved in a small amount of a volatile solvent, such as dichloromethane (CH2Cl2). A drop of this solution is placed on a single salt plate, and the solvent is allowed to evaporate.
This leaves behind a thin, solid film of your compound "cast" onto the plate. It is crucial to also run a spectrum of the pure solvent to identify and subtract any of its residual peaks from your sample's spectrum.
Preparing Solid Samples
Solid sample preparation requires mixing the compound into an IR-transparent matrix. The key is to grind the solid into a fine powder to reduce light scattering, which can distort the spectrum.
KBr Pellets
This is the most common method for high-quality solid-state spectra. A small amount of the sample (1-2 mg) is mixed with about 100-200 mg of very dry KBr powder.
The mixture is ground to an extremely fine, homogenous powder. It is then compressed under high pressure in a special die to form a small, transparent pellet that can be analyzed directly.
Nujol Mulls
A Nujol mull is an alternative when a sample is difficult to grind or may react with KBr. The solid is ground with a few drops of mineral oil (Nujol) to create a thick paste, or "mull."
This paste is then spread thinly between two salt plates, similar to a neat liquid sample. A key disadvantage is that the Nujol itself has C-H bond absorptions that will appear in the spectrum.
Preparing Gas Samples
Gases have a very low density of molecules compared to liquids or solids. To get a measurable signal, the IR beam must travel through a much longer path of the sample.
Long Path Length Gas Cells
Gases are analyzed using a sealed cell, typically 5 to 10 cm long, with IR-transparent windows (e.g., KBr or NaCl) at both ends. The cell is first evacuated and then filled with the gas sample. This long path length ensures enough molecules interact with the beam to produce a clear spectrum.
Understanding the Trade-offs
No method is perfect. Being aware of the potential pitfalls is key to interpreting your results correctly.
The Problem of Water
Because NaCl and KBr plates are hygroscopic, they can be easily damaged by moisture from the air, your hands, or your sample. They must be stored in a desiccator and handled with care. Any absorbed water will show up as a very broad, strong peak around 3200-3600 cm⁻¹, potentially obscuring important N-H or O-H signals.
Contamination from the Matrix
The matrix used to prepare your sample can introduce its own signals. Nujol will always show prominent C-H stretching and bending peaks. KBr must be of high purity and kept perfectly dry to avoid introducing water or other impurity peaks.
Incorrect Concentration
Proper preparation ensures the absorbance of your strongest peaks falls within the detector's optimal range. If the sample is too concentrated (e.g., the liquid film is too thick), the strongest peaks will be "flat-topped" or "cut off," making them useless for quantitative analysis. If it is too dilute, the signal will be weak and noisy.
Making the Right Choice for Your Sample
Your sample's physical state dictates the best preparation method.
- If you have a pure, low-viscosity liquid: Use the neat thin film method between two salt plates for the purest spectrum.
- If you have a solid sample: The KBr pellet method generally provides the highest quality spectrum with no background interference.
- If your solid is sensitive to pressure or moisture: A Nujol mull is a fast and effective alternative, but you must disregard the known Nujol peaks in your analysis.
- If you have a gas or highly volatile compound: A long path length gas cell is the only appropriate method.
- If your sample is only available as a solution: Use a cast film or liquid cell, and remember to run a background spectrum of the pure solvent for subtraction.
Ultimately, mastering sample preparation is the most critical step toward obtaining a clear and meaningful IR spectrum.
Summary Table:
| Sample State | Primary Method | Key Consideration |
|---|---|---|
| Solid | KBr Pellet or Nujol Mull | Grind finely to reduce light scattering. |
| Liquid | Neat Thin Film or Solution Cast | Ensure a thin, uniform layer for analysis. |
| Gas | Long Path Length Cell | Provides sufficient molecule density for detection. |
Obtain clear, accurate IR spectra with the right sample preparation tools from KINTEK.
Proper sample preparation is the foundation of successful Infrared (IR) spectroscopy. Whether you are analyzing solids, liquids, or gases, using the correct method and high-quality materials is essential to avoid interference and ensure reliable results.
KINTEK specializes in laboratory equipment and consumables for all your IR spectroscopy needs. We provide the essential tools for precise sample preparation:
- High-Purity Salt Plates (KBr, NaCl) for liquid and mull analysis.
- KBr Pellet Dies for creating high-quality solid samples.
- Durable Gas Cells with long path lengths for volatile compounds.
Our products help you achieve optimal sample concentration and transparency, leading to clear, interpretable spectra free from common pitfalls like water contamination or detector saturation.
Ready to enhance your lab's IR analysis? Contact our experts today to find the perfect sample preparation solution for your specific application.
Visual Guide
Related Products
- Laboratory Hydraulic Press Split Electric Lab Pellet Press
- Laboratory Hydraulic Press Lab Pellet Press for Button Battery
- Automatic Laboratory Hydraulic Press for XRF & KBR Pellet Press
- Automatic Laboratory Hydraulic Pellet Press Machine for Lab Use
- Laboratory Hydraulic Pellet Press for XRF KBR FTIR Lab Applications
People Also Ask
- What role does an open reactor play in the SHS process? Enhance Your Surface Coatings Today
- What is an example of a continuous furnace? Discover the Conveyor Belt Furnace for High-Volume Production
- Is biomass a sustainable energy option? Unlocking a Truly Sustainable Energy Future
- What units are used for heat capacity? A Guide to J/K, J/(kg·K), and J/(mol·K)
- What are the advantages of RF sputtering over DC sputtering? Achieve Superior Thin Films for Advanced Applications
- What is the temperature range of quartz glass? Master Its Thermal Limits for Demanding Applications
- What is in pyrolysis oil? Unlocking the Complex Chemistry of Bio-Oil
- What is heat treatment in simple terms? A Guide to Transforming Material Properties



















