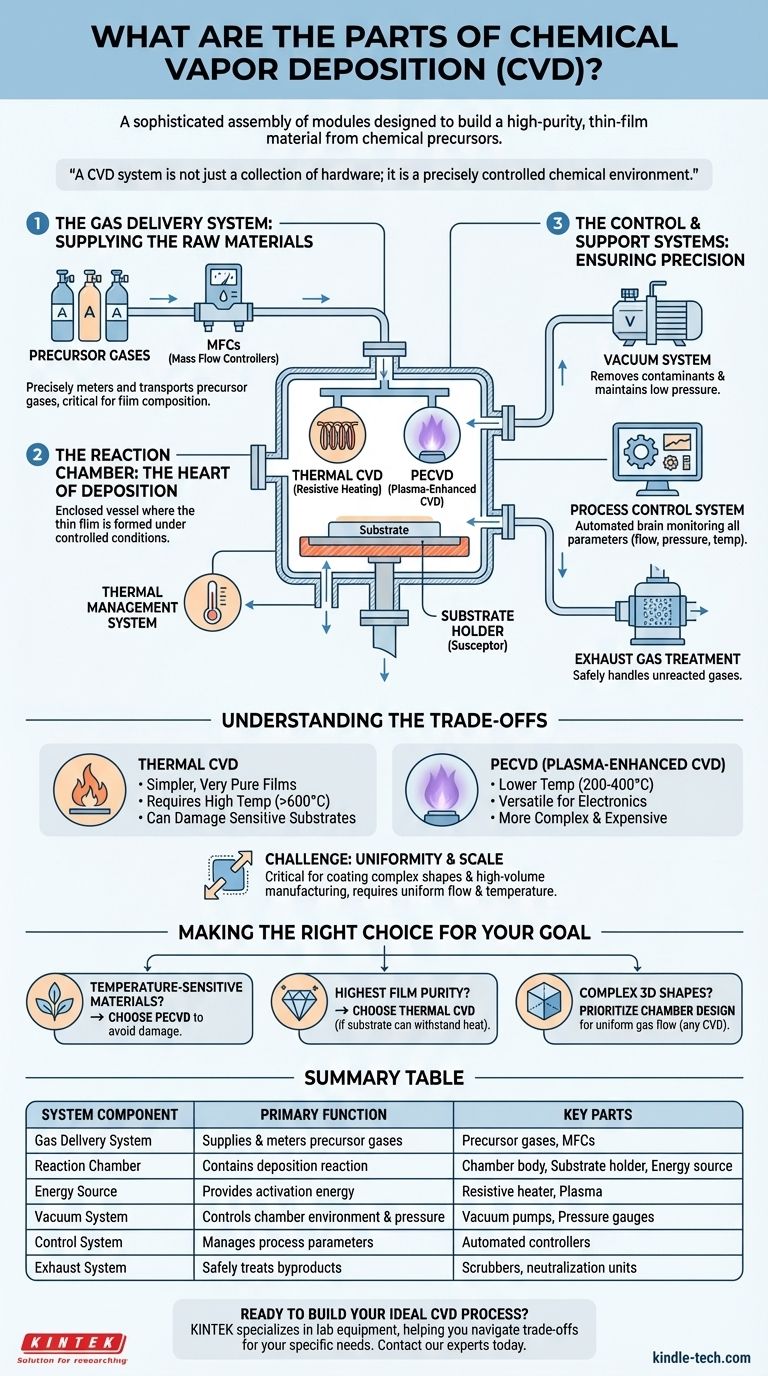
At its core, a Chemical Vapor Deposition (CVD) system is a sophisticated assembly of modules designed to build a high-purity, thin-film material from chemical precursors. The primary hardware components include a gas delivery system, a reaction chamber, an energy source to drive the reaction, a vacuum system to control the environment, and a control system to manage the entire process. These parts work in unison to facilitate a chemical reaction that deposits a solid material onto a substrate surface.
A CVD system is not just a collection of hardware; it is a precisely controlled chemical environment. The core components work together to introduce reactive gases (precursors), apply energy to break them down, and enable the deposition of a new, solid layer onto a target surface with atomic-scale precision.

The Key Functional Systems of CVD
We can group the physical parts of a CVD system into three primary functional areas: the systems that introduce the raw materials, the environment where the reaction occurs, and the systems that control and support the entire process.
The Gas Delivery System: Supplying the Raw Materials
The process begins with the precursor gases, which are the chemical building blocks for the final film.
The gas delivery system is responsible for precisely metering and transporting these gases into the reaction chamber. This is far more than simple plumbing; it involves mass flow controllers (MFCs) that ensure the exact ratio of different gases is maintained, which is critical for the final film's chemical composition and quality.
The Reaction Chamber: The Heart of Deposition
This is the central component where the thin film is actually formed.
The reaction chamber is an enclosed vessel designed to contain the chemical reaction under highly controlled conditions. Inside the chamber is a holder, often called a susceptor or stage, where the substrate (the material to be coated) is placed.
A crucial part of this system is the energy source. This is what provides the activation energy needed to break down the precursor gases and initiate the deposition. The type of energy source used often defines the specific type of CVD, such as using resistive heating for Thermal CVD or plasma for Plasma-Enhanced CVD (PECVD).
Finally, the thermal management system is responsible for heating the substrate to a specific temperature. Substrate temperature is a critical variable that directly influences the deposition rate and the structural properties of the resulting film.
The Control and Support Systems: Ensuring Precision
These ancillary systems are what make the CVD process reliable and repeatable.
The vacuum system, typically consisting of one or more pumps, serves two purposes. It first removes all atmospheric gases and contaminants from the chamber, creating a pure environment. It then maintains the chamber at a specific low pressure, which affects how the gas molecules travel and react.
The process control system acts as the brain of the entire operation. This automated system monitors and adjusts all critical parameters—gas flow rates, chamber pressure, and substrate temperature—to ensure the process runs exactly as intended from start to finish.
An exhaust gas treatment system safely handles the unreacted precursor gases and chemical byproducts, neutralizing them before they are released.
Understanding the Trade-offs
The choice and configuration of these components are not arbitrary; they represent critical trade-offs between process capability, cost, and material compatibility.
The Impact of the Energy Source
The most significant trade-off often involves the energy source. A thermal CVD system is simpler and can produce very pure films, but it requires extremely high temperatures (often >600°C) that can damage or warp sensitive substrates like plastics or certain electronic components.
In contrast, PECVD uses an electrical field to create plasma, which provides the energy to break down the precursors at much lower temperatures (200-400°C). This makes it highly versatile for modern electronics, but the equipment is more complex and expensive.
The Challenge of Uniformity and Scale
While CVD is excellent for coating complex shapes due to its non-line-of-sight nature, achieving a perfectly uniform film thickness is a significant engineering challenge. The design of the reaction chamber, the gas flow dynamics, and the temperature consistency across the entire substrate are all critical factors.
Scaling the process up for high manufacturing yield requires an even more sophisticated level of control over these variables to ensure every part is coated identically.
Making the Right Choice for Your Goal
The ideal CVD setup depends entirely on the material you are depositing and the substrate you are coating.
- If your primary focus is depositing on temperature-sensitive materials: A system with a plasma-based energy source (PECVD) is the necessary choice to avoid damaging the substrate.
- If your primary focus is achieving the highest film purity at a lower equipment cost: A traditional thermal CVD system is often sufficient, provided your substrate can withstand the heat.
- If your primary focus is coating complex, three-dimensional shapes: The non-line-of-sight nature of any CVD process is a key advantage, but you must prioritize a well-designed reaction chamber for uniform gas flow.
By understanding how these core components interact, you can effectively control the chemical reaction to achieve your specific material and performance goals.
Summary Table:
| System Component | Primary Function | Key Parts |
|---|---|---|
| Gas Delivery System | Supplies and meters precursor gases | Precursor gases, Mass Flow Controllers (MFCs) |
| Reaction Chamber | Contains the deposition reaction | Chamber body, Substrate holder (susceptor), Energy source |
| Energy Source | Provides activation energy for the reaction | Resistive heater (Thermal CVD), Plasma (PECVD) |
| Vacuum System | Controls chamber environment and pressure | Vacuum pumps, Pressure gauges |
| Control System | Manages process parameters for repeatability | Automated controllers for temperature, pressure, gas flow |
| Exhaust System | Safely treats byproducts and unused gases | Scrubbers, neutralization units |
Ready to Build Your Ideal CVD Process?
Understanding the components is the first step. Implementing the right system for your specific material and substrate is the next. KINTEK specializes in lab equipment and consumables, providing the precise CVD solutions your laboratory needs.
We can help you navigate the trade-offs between thermal and plasma-enhanced systems to achieve your goals, whether you need high-purity films or low-temperature deposition on sensitive materials.
Contact our experts today to discuss how we can support your thin-film research and production with reliable, high-performance equipment.
Visual Guide
Related Products
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- Rotary Tube Furnace Split Multi Heating Zone Rotating Tube Furnace
People Also Ask
- What is CVD method chemical Vapour deposition? The Process for High-Purity Thin Films
- How do you manufacture lab-grown diamonds? Discover the HPHT and CVD Methods
- What is the difference between physical Vapour deposition and chemical Vapour deposition? Choose the Right Thin-Film Coating Process
- What is the thermal vapor deposition technique? A Guide to PVD and CVD Coating Methods
- What critical environmental conditions does a high-temperature furnace provide for CVD aluminization? Master 1050°C Precision
- What is the thermal CVD technique? The High-Temperature Secret to Superior Coatings
- What is CVD method for synthesis of nanomaterials? A Guide to Atomic-Level Material Fabrication
- What methods are used to deposit thin films? A Guide to PVD, CVD, and ALD Techniques



















