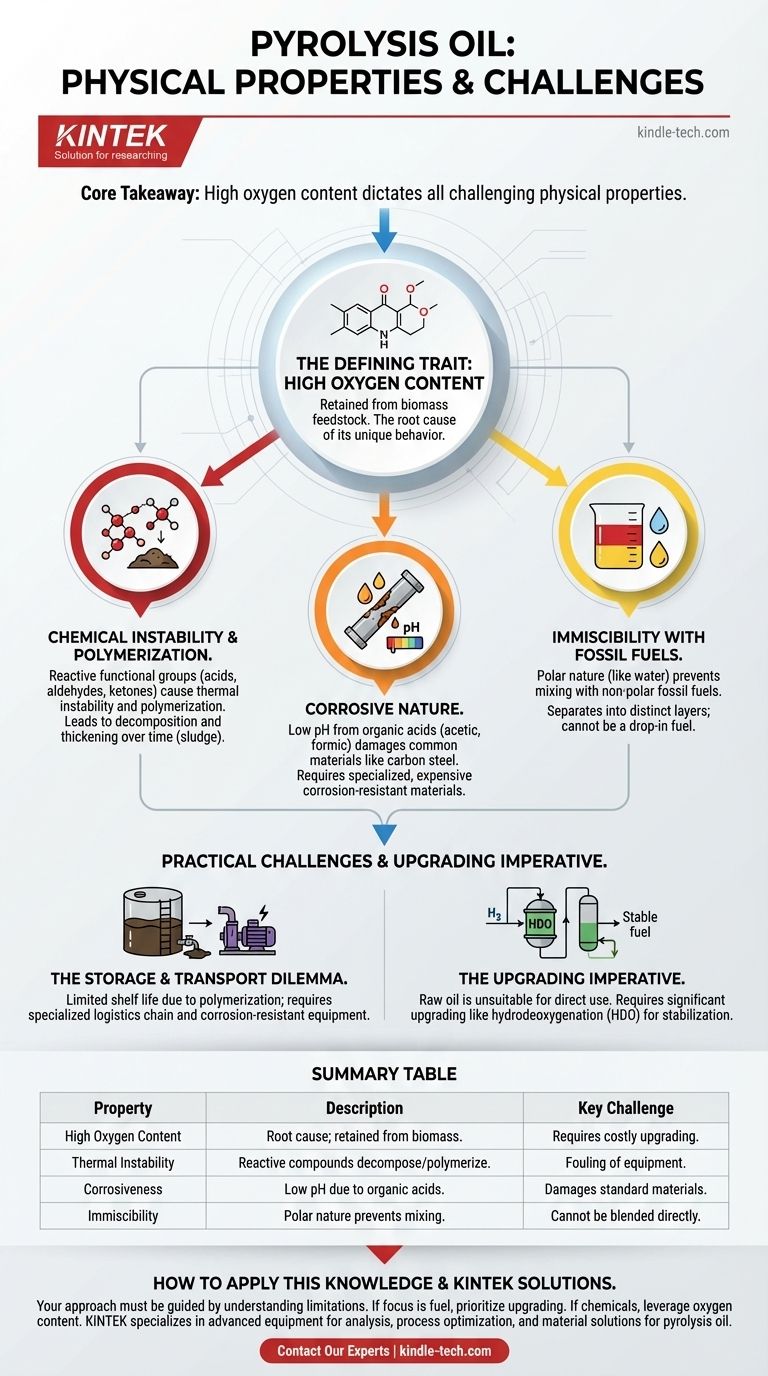
Pyrolysis is a thermal decomposition process, and the physical properties you're likely asking about belong to its primary liquid product: pyrolysis oil, also known as bio-oil. Unlike conventional petroleum, pyrolysis oil is defined by a high oxygen content, which makes it corrosive, thermally unstable, and immiscible with fossil fuels. These characteristics present significant challenges for its use as a direct fuel replacement.
The core takeaway is that the high oxygen content of pyrolysis oil dictates all of its other challenging physical properties. This makes it fundamentally different from petroleum and means it must be treated as a crude, reactive intermediate that requires significant upgrading, not as a finished, drop-in fuel.

The Defining Trait: High Oxygen Content
The source material for pyrolysis—biomass—contains a significant amount of oxygen. While pyrolysis breaks down large organic polymers, much of this oxygen is retained in the resulting liquid oil. This single chemical difference is the root cause of its unique and often problematic physical behavior compared to crude petroleum, which is composed almost entirely of hydrocarbons (hydrogen and carbon).
Chemical Instability and Polymerization
The oxygen in pyrolysis oil exists within reactive functional groups like acids, aldehydes, and ketones. These compounds are not stable.
This inherent reactivity leads to thermal instability. When heated, as it would be in an engine or refinery, the oil can decompose further or react in unpredictable ways, potentially fouling equipment.
It also causes polymerization. When exposed to air or simply over time, the small reactive molecules in the oil can link together to form larger ones. This process thickens the oil, increasing its viscosity and eventually forming sludge and solid deposits.
Corrosive Nature
A significant portion of the oxygen in bio-oil is found in organic acids, such as acetic and formic acid. This gives the oil a low pH, making it highly corrosive.
This acidity can damage common construction materials like carbon steel and certain elastomers used in seals and gaskets. Handling, storing, and transporting pyrolysis oil requires more expensive, corrosion-resistant materials like stainless steel.
Immiscibility with Fossil Fuels
The oxygen-containing compounds make pyrolysis oil a polar liquid, similar to water. In contrast, fossil fuels like gasoline and diesel are non-polar.
Based on the chemical principle of "like dissolves like," polar and non-polar liquids do not mix. This means pyrolysis oil is immiscible with conventional fuels, preventing simple blending to create hybrid fuels. They will separate into distinct layers, much like oil and water.
Understanding the Practical Challenges
These physical properties are not just academic; they have direct, real-world consequences for how pyrolysis oil can be used. Treating it like conventional crude oil will lead to significant operational failures.
The Storage and Transport Dilemma
The oil's tendency to polymerize gives it a limited shelf life. It can degrade during storage, becoming thicker and harder to pump, and potentially damaging equipment.
Furthermore, its corrosive nature means that the entire logistics chain—from storage tanks and pumps to transport tankers—must be constructed from specialized, corrosion-resistant materials, which adds significant cost and complexity.
The Upgrading Imperative
Because of its instability, corrosiveness, and immiscibility, raw pyrolysis oil cannot be used as a "drop-in" fuel in existing engines or refineries.
It must first undergo a significant upgrading process, most commonly hydrodeoxygenation (HDO). This process uses a catalyst and hydrogen at high pressure and temperature to remove oxygen atoms from the oil's molecules, creating a more stable, non-corrosive, hydrocarbon-like product that can be further refined.
How to Apply This Knowledge
Your approach to pyrolysis oil must be guided by a clear understanding of its inherent limitations and potential.
- If your primary focus is direct fuel replacement: Recognize that raw pyrolysis oil is unsuitable. You must factor in the necessity and cost of a dedicated upgrading process to de-oxygenate and stabilize the oil before it can be integrated with conventional fuel systems.
- If your primary focus is producing specialty chemicals: View the high oxygen content as a potential advantage. The oil is a rich source of oxygenated compounds that can be isolated and used as valuable feedstocks for the chemical industry, avoiding the need for costly de-oxygenation.
Understanding these fundamental properties is the critical first step in correctly evaluating the role of pyrolysis oil in a circular economy or renewable energy strategy.
Summary Table:
| Property | Description | Key Challenge |
|---|---|---|
| High Oxygen Content | Retained from biomass feedstock; the root cause of other properties. | Requires costly upgrading (e.g., HDO) for stability. |
| Thermal Instability | Reactive compounds decompose or polymerize when heated. | Fouling of engines and refinery equipment. |
| Corrosiveness | Low pH due to organic acids (e.g., acetic acid). | Damages standard materials; requires stainless steel. |
| Immiscibility | Polar nature prevents mixing with non-polar fossil fuels. | Cannot be directly blended as a drop-in fuel. |
Ready to navigate the complexities of pyrolysis oil?
Understanding the challenging physical properties of pyrolysis oil is the first step toward developing a successful strategy for its use, whether for biofuel production or chemical extraction. At KINTEK, we specialize in providing the advanced laboratory equipment and consumables needed to analyze, test, and upgrade pyrolysis products effectively.
Our expertise supports researchers and engineers in:
- Precise Analysis: Accurately characterizing pyrolysis oil properties.
- Process Optimization: Developing efficient upgrading techniques like hydrodeoxygenation (HDO).
- Material Solutions: Supplying corrosion-resistant equipment for handling reactive bio-oils.
Let KINTEK be your partner in transforming challenging biomass derivatives into viable resources. Contact our experts today to discuss your specific laboratory needs and discover how our solutions can drive your project forward.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Heating Pyrolysis Plant
- Thin-Layer Spectral Electrolysis Electrochemical Cell
- Custom PTFE Teflon Parts Manufacturer for Culture Dish and Evaporation Dish
- Custom PTFE Teflon Parts Manufacturer for PTFE Tweezers
People Also Ask
- Why must cold traps and drying tubes be configured for WGS gas analysis? Protect Your Micro-GC from Moisture Damage.
- Does pyrolysis produce greenhouse gases? Discover Its Net Role in Reducing Emissions
- What is the difference between laser melting and sintering? A Guide to Particle Fusion Methods
- How does a controlled drying process ensure the quality of radiochromic films? Achieve Precise Dosimetric Results
- Is natural or synthetic graphite better? Choosing the Right Material for Your Application
- What are some additional advantages of using Ultra-Low Temperature freezers in laboratories? Boost Lab Efficiency and Cut Costs
- What is the use of pyrolysis fuel? A Sustainable Substitute for Industrial Heating and Power
- Does higher heat capacity mean higher melting point? Unraveling the Critical Difference





