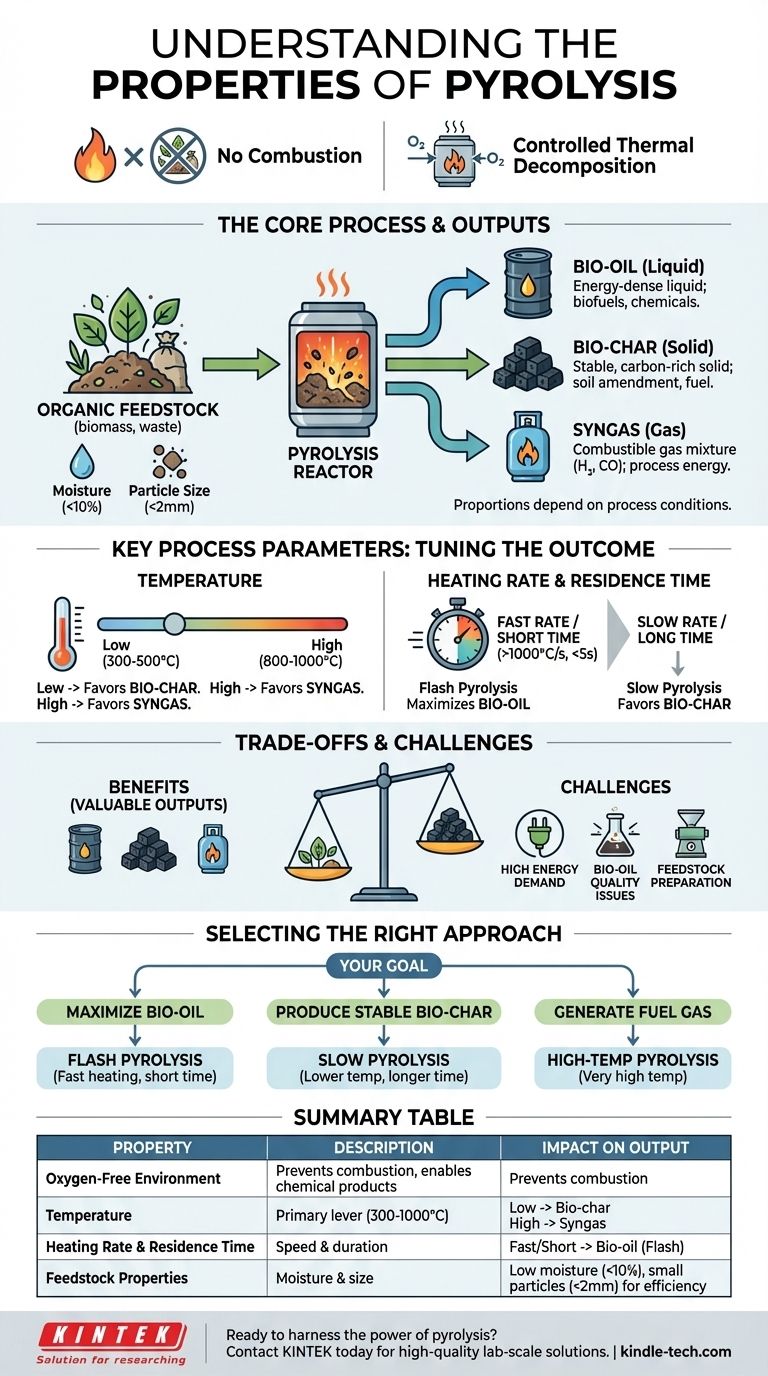
At its core, pyrolysis is the thermochemical decomposition of organic material by applying high heat in a completely oxygen-free environment. Unlike burning, which uses oxygen to produce flame, ash, and smoke, pyrolysis chemically breaks down materials into three distinct and valuable outputs: a liquid known as bio-oil, a solid called bio-char, and a combustible gas mixture called syngas. The specific properties of the process are defined by the parameters you control.
Pyrolysis should not be viewed as a single process, but as a highly tunable platform. Its defining property is the controlled application of heat without oxygen, which allows you to precisely manipulate variables like temperature and heating rate to selectively produce either liquid fuel, solid carbon, or combustible gas from a waste stream.

The Defining Mechanism: Heat Without Combustion
Pyrolysis functions by breaking complex organic polymers into simpler, smaller molecules. The absence of oxygen is the single most critical property of the process, as it prevents the material from combusting and instead forces it to thermally decompose.
The Three Core Products
The process yields a predictable set of outputs. The proportion of each depends entirely on the process conditions.
- Bio-oil (Liquid): An energy-dense liquid that can be used for producing heat, power, or be upgraded into advanced biofuels and chemicals.
- Bio-char (Solid): A stable, carbon-rich solid similar to charcoal. It can be used as a soil amendment, for filtration, or as a solid fuel.
- Syngas (Gas): A mixture of combustible gases, primarily hydrogen and carbon monoxide. It can be burned on-site to provide the energy needed to run the pyrolysis process itself, making the system more self-sufficient.
Why No Oxygen is the Critical Factor
If oxygen were present, the organic material would simply burn (combust), releasing its energy as heat and producing carbon dioxide and water.
By eliminating oxygen, pyrolysis preserves the chemical energy within the feedstock, repackaging it into the chemical bonds of the bio-oil, bio-char, and syngas products.
Key Process Parameters That Define the Outcome
The "properties" of a specific pyrolysis operation are a direct result of the conditions chosen. By adjusting these levers, you can steer the reaction to favor one output over another.
Temperature
Temperature is a primary driver of the final product mix. Lower temperatures (300-500°C) and slower processes favor the production of solid bio-char.
Conversely, very high temperatures (800-1000°C) tend to "crack" the larger molecules further, maximizing the yield of syngas.
Heating Rate & Residence Time
This refers to how quickly the material is heated and how long the resulting vapors remain in the hot reactor zone.
Flash pyrolysis, for example, uses an extremely high heating rate (>1000°C/s) and a very short vapor residence time (<5 seconds). These conditions are specifically designed to maximize the liquid bio-oil yield, often achieving up to 75% of the product mass.
Feedstock Characteristics
The input material itself has properties that influence the process. The two most important are moisture content and particle size.
An ideal moisture content is around 10%. Higher moisture requires more energy to evaporate the water, reducing efficiency. Small particle sizes (typically under 2 mm) are essential for ensuring the material heats rapidly and evenly.
Understanding the Trade-offs and Challenges
While powerful, pyrolysis is not a silver bullet. Understanding its limitations is critical for successful implementation.
The High Energy Demand
Bringing feedstock to temperatures of 400-900°C is an energy-intensive process. While the syngas produced can offset some of this demand, the initial energy input remains a significant operational consideration.
The Problem with Bio-Oil
Pyrolysis-derived bio-oil is not a direct replacement for petroleum products. Due to its high oxygen content, it is inherently corrosive, thermally unstable, and does not mix with conventional fossil fuels. It often requires significant and costly upgrading before it can be used in standard engines or refineries.
The Need for Feedstock Preparation
You cannot simply feed raw waste into most pyrolysis reactors. The material must be dried to the correct moisture level and ground to a uniform, small particle size, adding cost and complexity to the overall operation.
How to Select the Right Pyrolisis Approach
Your end goal dictates the ideal process parameters. The flexibility of pyrolysis is its greatest strength, allowing for a wide range of applications from waste reduction to targeted chemical production.
- If your primary focus is maximizing liquid fuel (bio-oil): You need a fast or flash pyrolysis setup with very high heating rates and short vapor residence times.
- If your primary focus is producing a stable solid (bio-char): You need a slow pyrolysis process with lower temperatures and a much longer processing time.
- If your primary focus is generating fuel gas (syngas): You need a process that operates at very high temperatures to ensure all volatile components are broken down into simple gas molecules.
Ultimately, mastering pyrolysis is about understanding how to precisely control its operating conditions to transform a specific waste stream into your desired valuable product.
Summary Table:
| Property | Description | Impact on Output |
|---|---|---|
| Oxygen-Free Environment | The defining feature; prevents combustion, enabling thermal decomposition. | Enables production of valuable chemical products instead of just heat/ash. |
| Temperature | The primary control lever (typically 300-1000°C). | Lower temps favor bio-char; higher temps favor syngas. |
| Heating Rate & Residence Time | Speed of heating and time vapors spend in the hot zone. | Fast rates/short times maximize bio-oil (flash pyrolysis). |
| Feedstock Properties | Moisture content and particle size of the input material. | Low moisture (<10%) and small particles (<2mm) are ideal for efficiency. |
Ready to Harness the Power of Pyrolysis in Your Lab?
Mastering the properties of pyrolysis requires precise control and reliable equipment. At KINTEK, we specialize in high-quality lab-scale pyrolysis systems that allow you to precisely tune temperature, heating rate, and atmosphere to transform your specific biomass or waste materials into valuable products like bio-oil, bio-char, or syngas.
Our reactors are designed for researchers and developers focused on waste valorization, renewable energy, and sustainable materials. Let us provide the robust equipment you need to optimize your process and achieve your conversion goals.
Contact KINTEK today to discuss your pyrolysis application and find the perfect solution for your laboratory.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Electric Rotary Kiln Pyrolysis Furnace Plant Machine Calciner Small Rotary Kiln Rotating Furnace
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Heating Pyrolysis Plant
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Electric Rotary Kiln Small Rotary Furnace for Activated Carbon Regeneration
People Also Ask
- What is a disadvantage of biomass energy? The Hidden Environmental and Economic Costs
- Is pyrolysis viable? A Guide to Economic, Technological, and Environmental Success
- What is the process of biomass fast pyrolysis? Turn Biomass into Bio-Oil in Seconds
- How is energy converted into biomass? Harnessing Nature's Solar Power for Renewable Energy
- What are the advantages of pyrolysis technology? Turn Waste into Profit and Reduce Emissions



















