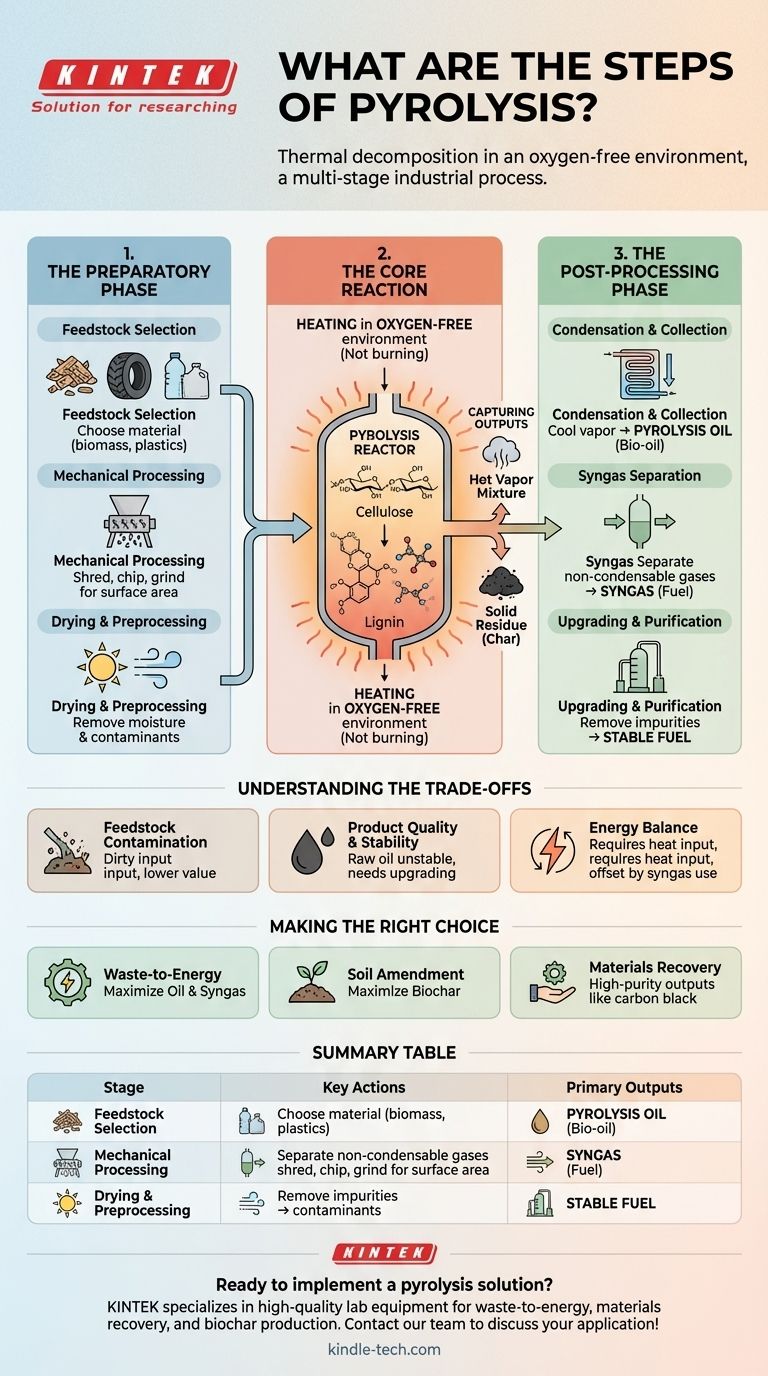
At its core, pyrolysis is a multi-stage process of thermal decomposition that breaks down material in an oxygen-free environment. It consists of three main phases: preparing the raw material (feedstock), the central heating reaction itself, and finally, the collection and refinement of the resulting products like oil, gas, and a solid residue called char.
Pyrolysis is best understood not as a single event, but as a complete industrial process. Success depends just as much on the initial preparation of materials and the final purification of products as it does on the core chemical reaction.
The Preparatory Phase: Getting the Feedstock Ready
Before any heating occurs, the raw material must be carefully prepared to ensure an efficient and clean reaction. This initial stage is critical for maximizing the yield of valuable products.
Feedstock Selection
The process begins by choosing a suitable material, known as feedstock. Common feedstocks include biomass (like wood or agricultural waste), plastics, and used tires.
Mechanical Processing
The feedstock is typically shredded, chipped, or ground into smaller, more uniform pieces. This increases the surface area, allowing heat to penetrate the material more evenly and quickly during the reaction.
Drying and Preprocessing
Moisture is a significant inhibitor of efficient pyrolysis, so the feedstock is thoroughly dried. This stage also involves removing contaminants, such as metals or other non-target materials, to prevent unwanted chemical reactions and ensure product purity.
The Core Reaction: Thermal Decomposition
This is the heart of the pyrolysis process, where heat performs its work in a controlled, oxygen-deprived environment. The name itself comes from the Greek pyro (fire) and lysis (separation).
The Pyrolysis Reactor
The prepared feedstock is fed into a sealed vessel called a reactor. All oxygen is purged from this system, which is the defining characteristic of pyrolysis; the material is heated, not burned.
Heating and Decomposition
The reactor is heated to high temperatures, causing the long-chain molecules within the feedstock to vibrate violently and break apart into smaller, simpler molecules. For biomass, this involves the decomposition of its main components: cellulose, hemicellulose, and lignin.
Capturing the Outputs
As the material decomposes, it releases a hot mixture of gases and vapors. This mixture is immediately drawn out of the reactor for the next phase, leaving behind a solid, carbon-rich residue known as biochar (from biomass) or carbon black (from plastics/tires).
The Post-Processing Phase: Refining the Products
The raw output from the reactor is a mix of compounds that must be separated and refined to become usable products.
Condensation and Collection
The hot vapor stream is rapidly cooled in a condenser. The components with higher boiling points turn back into a liquid, which is collected as pyrolysis oil (or bio-oil).
Syngas Separation
The remaining, non-condensable gases are separated out. This product, known as syngas (synthesis gas), is typically rich in hydrogen and carbon monoxide and can be used as a fuel to power the pyrolysis process itself.
Upgrading and Purification
The raw pyrolysis oil is often acidic, unstable, and contains oxygen or other impurities. It must undergo an upgrading process, such as distillation or other chemical treatments, to remove these elements and stabilize it for use as a commercial fuel.
Understanding the Trade-offs
While powerful, pyrolysis is a sensitive process with critical variables that must be managed.
Feedstock Contamination
The quality of the final products is directly tied to the purity of the initial feedstock. Contaminants can introduce unwanted chemicals into the final oil or char, reducing their value and potentially creating hazardous byproducts.
Product Quality and Stability
Raw pyrolysis oil is not a "drop-in" replacement for conventional fossil fuels. It requires the energy-intensive post-processing and upgrading steps to become a stable, usable product.
Energy Balance
Pyrolysis requires a significant energy input to reach and maintain its high operating temperatures. The overall efficiency of a system depends on its ability to use the syngas it produces to offset its own energy consumption.
Making the Right Choice for Your Goal
The specific parameters of the pyrolysis process are adjusted based on the desired outcome.
- If your primary focus is waste-to-energy: The process is optimized to maximize the yield and quality of the liquid pyrolysis oil and combustible syngas.
- If your primary focus is soil amendment: Operating conditions are tailored to produce a high yield of stable, nutrient-rich biochar.
- If your primary focus is materials recovery: The goal is to create high-purity outputs, such as recovering carbon black from tires for reuse in manufacturing.
Understanding these distinct stages reveals pyrolysis as a versatile platform for converting low-value materials into valuable resources.
Summary Table:
| Stage | Key Actions | Primary Outputs |
|---|---|---|
| 1. Preparation | Feedstock selection, shredding, drying, decontamination | Clean, dry, uniform feedstock |
| 2. Core Reaction | Heating in an oxygen-free reactor | Hot vapor mixture & solid char |
| 3. Post-Processing | Condensation, gas separation, oil upgrading | Pyrolysis oil, syngas, purified biochar |
Ready to implement a pyrolysis solution for your laboratory or facility? KINTEK specializes in high-quality lab equipment and consumables for advanced thermal processes. Whether your goal is waste-to-energy conversion, materials recovery, or biochar production, our experts can help you select the right reactor and purification systems to maximize your yield and efficiency. Contact our team today to discuss your specific application and get a tailored solution!
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Heating Pyrolysis Plant
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- Is pyrolysis viable? A Guide to Economic, Technological, and Environmental Success
- What are the reactions involved in pyrolysis of biomass? Unlock the Chemistry for Tailored Bio-Products
- What are the components of biomass pyrolysis? A Complete Guide to the System, Products, and Process
- What is a disadvantage of biomass energy? The Hidden Environmental and Economic Costs
- What is the process of biomass fast pyrolysis? Turn Biomass into Bio-Oil in Seconds



















