The primary techniques of Chemical Vapor Deposition (CVD) include Thermal CVD, Plasma-Enhanced CVD (PECVD), and Metalorganic CVD (MOCVD), among others. These methods are differentiated by the energy source used to drive the chemical reaction—such as heat or plasma—and the specific type of chemical precursor delivered to the substrate.
The core principle to understand is that all CVD techniques are simply different tools to solve the same problem: initiating a chemical reaction in a gas phase to create a high-quality solid film on a surface. The choice of technique is a strategic decision based on the required film properties, the substrate's temperature tolerance, and production cost.

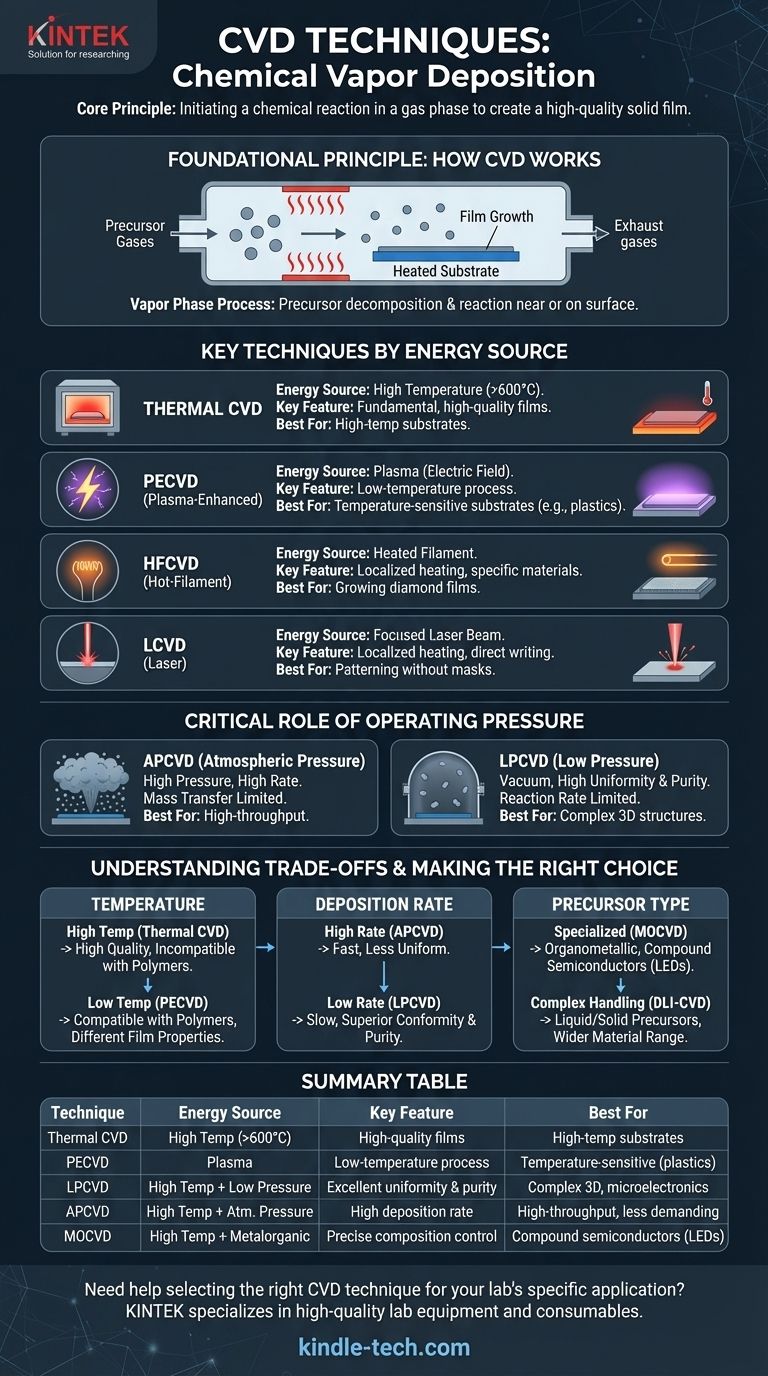
The Foundational Principle: How CVD Works
Chemical Vapor Deposition is a process used to create thin, solid films on a substrate, which is a foundational technique in manufacturing semiconductors, optics, and advanced materials.
The Core Process
The process involves introducing one or more volatile precursor gases into a reaction chamber. These gases decompose and react near or on a heated substrate surface, leading to the deposition of a thin film of the desired material.
Defining CVD's Place
It's crucial to distinguish CVD from other deposition methods. While processes like plating and sol-gel are forms of Chemical Deposition, they occur in a liquid solution. CVD is distinct because the entire process—from precursor transport to reaction—happens in the vapor or gas phase.
Key CVD Techniques Categorized by Energy Source
The most effective way to understand different CVD methods is by looking at how they supply the energy needed to break down the precursor gases and initiate the deposition reaction.
Thermal CVD
Thermal CVD is the most fundamental technique. It relies solely on high temperatures (often >600°C) to provide the thermal energy required for the chemical reaction to occur on the substrate surface.
Plasma-Enhanced CVD (PECVD)
Plasma-Enhanced CVD (PECVD) uses an electric field to generate a plasma (a high-energy ionized gas). This plasma provides the energy to break apart the precursor molecules, allowing deposition to occur at much lower temperatures than thermal CVD. This makes it ideal for substrates that cannot withstand high heat.
Hot-Filament CVD (HFCVD)
A variation of thermal CVD, Hot-Filament CVD (HFCVD) uses a heated filament placed near the substrate to thermally decompose the precursor gases. This localized heating is efficient for specific materials, like growing diamond films.
Laser CVD (LCVD)
Laser CVD (LCVD) uses a focused laser beam to heat a very small, specific area of the substrate. This localized heating drives the deposition reaction only where the laser is pointed, allowing for direct writing or patterning of materials without masks.
The Critical Role of Operating Pressure
Beyond the energy source, the pressure inside the reaction chamber is a fundamental variable that dictates the deposition process and the final film quality.
Atmospheric Pressure CVD (APCVD)
This technique operates at normal atmospheric pressure. It allows for high deposition rates and is relatively simple, but the film uniformity and purity can be lower because the reaction is limited by how fast precursor gases can travel through the dense atmosphere to the surface (mass transfer limited).
Low-Pressure CVD (LPCVD)
LPCVD is performed in a vacuum (low pressure). The reduced pressure allows gas molecules to move freely, ensuring the reaction rate is limited only by the chemical reactions on the substrate surface itself (reaction rate limited). This results in films with excellent uniformity and purity, even on complex 3D structures.
Understanding the Trade-offs
Choosing a CVD technique always involves balancing competing factors. There is no single "best" method; the optimal choice depends entirely on the application's specific requirements.
Temperature vs. Substrate Compatibility
The primary trade-off is between temperature and material choice. Thermal CVD produces high-quality films but is incompatible with temperature-sensitive materials like polymers. PECVD solves this by enabling low-temperature deposition, though the film properties might differ slightly.
Deposition Rate vs. Film Quality
APCVD offers fast deposition rates suitable for high-throughput manufacturing. However, this speed often comes at the cost of film uniformity. LPCVD is slower but provides superior conformity and purity, which is critical for high-performance microelectronics.
Precursor Type and Complexity
Some materials require specialized precursors. Metalorganic CVD (MOCVD) uses organometallic compounds, which are essential for creating high-quality compound semiconductor films for LEDs and lasers. Techniques like Direct Liquid Injection (DLI-CVD) are designed to handle precursors that are liquid or solid at room temperature, adding complexity but expanding the range of possible materials.
Making the Right Choice for Your Goal
Your application's primary driver will determine the most suitable CVD technique.
- If your primary focus is high purity and uniform coating on complex shapes: LPCVD is the superior choice due to its reaction-rate-limited nature.
- If your primary focus is depositing on a temperature-sensitive substrate like plastic: PECVD is the only viable option, as it replaces high heat with plasma energy.
- If your primary focus is high-speed, cost-effective production for less-demanding applications: APCVD provides the necessary throughput.
- If your primary focus is creating advanced compound semiconductor devices: MOCVD is the industry standard due to its precise control over composition.
Ultimately, selecting the right CVD technique is about matching the process characteristics to the specific demands of your final product.
Summary Table:
| Technique | Energy Source | Key Feature | Best For |
|---|---|---|---|
| Thermal CVD | High Temperature (>600°C) | High-quality films | High-temperature substrates |
| PECVD | Plasma | Low-temperature process | Temperature-sensitive substrates (e.g., plastics) |
| LPCVD | High Temperature + Low Pressure | Excellent uniformity & purity | Complex 3D structures, microelectronics |
| APCVD | High Temperature + Atmospheric Pressure | High deposition rate | High-throughput, less demanding coatings |
| MOCVD | High Temperature + Metalorganic Precursors | Precise composition control | Compound semiconductors (LEDs, lasers) |
Need help selecting the right CVD technique for your lab's specific application?
KINTEK specializes in providing high-quality lab equipment and consumables for all your deposition needs. Our experts can help you choose the perfect solution to achieve the film properties, substrate compatibility, and production efficiency your research demands.
Contact us today to discuss your project and discover how KINTEK can enhance your laboratory's capabilities!
Visual Guide
Related Products
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
- Multi-zone Laboratory Tube Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What is a CVD tube furnace? A Complete Guide to Thin-Film Deposition
- How high of temperature do carbon nanotubes in air have the ability to sustain? Understanding the Oxidation Limit
- Why are carbon nanotubes important in industry? Unlocking Next-Generation Material Performance
- What are the methods of producing CNT? Scalable CVD vs. High-Purity Lab Techniques
- What are the main advantages of Chemical Vapor Deposition (CVD)? Achieve Precision Coating for Complex Geometries



















