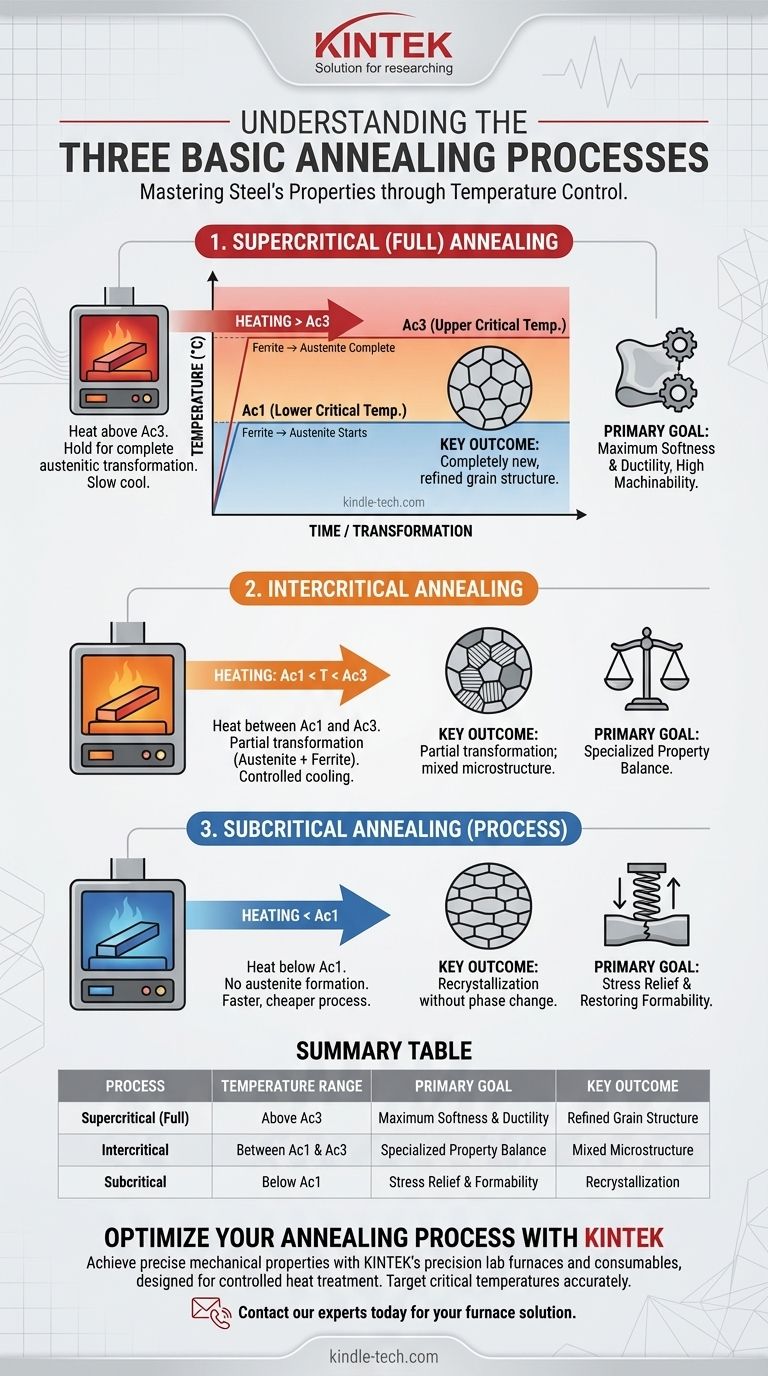
The three fundamental annealing processes are defined by the temperature to which the steel is heated relative to its critical transformation points. They are supercritical (or full) annealing, which heats the steel above its upper critical temperature (Ac3); intercritical annealing, which heats it between its lower (Ac1) and upper (Ac3) critical temperatures; and subcritical annealing, which heats it just below the lower critical temperature (Ac1).
The specific annealing process you choose is not arbitrary; it's a precise decision based on one key factor: temperature. Whether you heat the steel above, between, or below its critical transformation points directly dictates the change in its internal microstructure and, therefore, its final mechanical properties like softness, ductility, and machinability.
The Foundation: Steel's Critical Temperatures
To understand annealing, you must first understand the thermal "landmarks" within steel. These are not just numbers on a chart; they are temperatures at which the steel's crystal structure fundamentally reorganizes itself.
The Lower Critical Temperature (Ac1)
When heating a typical carbon steel, Ac1 is the temperature at which the initial structure of ferrite and cementite (pearlite) begins to transform into a new crystal structure called austenite.
The Upper Critical Temperature (Ac3)
As you continue heating past Ac1, more of the steel transforms. Ac3 is the temperature at which this transformation is complete, and the entire microstructure consists of 100% austenite.
Why These Temperatures Matter
Crossing these temperature boundaries is the entire mechanism of annealing. The process works by erasing the steel's existing microstructure (like a coarse, brittle structure from casting or a hardened structure from work-hardening) and forming a new, more desirable one upon slow cooling.
A Closer Look at the Three Core Processes
Each of the three basic processes uses these critical temperatures to achieve a different outcome.
Supercritical (Full) Annealing
This process involves heating the steel above the Ac3 temperature, holding it there long enough for the entire part to transform into a uniform austenitic structure.
The subsequent slow cooling allows a completely new, refined, and uniform grain structure of ferrite and pearlite to form. This yields the softest, most ductile, and most stress-free state possible, making the steel highly machinable. When an engineer simply says "annealing," they are typically referring to full annealing.
Intercritical Annealing
As the name implies, this process involves heating the steel to a temperature between Ac1 and Ac3.
This results in a partial transformation, creating a mixed microstructure of new austenite alongside some of the original ferrite. This process is less common but can be used to achieve specific intermediate properties that are not as soft as a full anneal.
Subcritical Annealing
This process involves heating the steel to a temperature just below the Ac1 point.
Because the temperature never reaches the first critical point, no austenite is formed. The primary goal here is not to create a new grain structure but to relieve internal stresses and allow for the recrystallization of ferrite grains that were distorted during cold working. This is often called process annealing or stress-relief annealing.
Understanding the Trade-offs
Choosing a process requires balancing metallurgical goals with practical constraints like time and cost.
Time and Energy Costs
Full (supercritical) annealing requires the highest temperatures and often the longest, most controlled cooling cycles. This makes it the most time-consuming and energy-intensive of the three basic types. Subcritical annealing is significantly faster and cheaper.
Final Hardness vs. Ductility
The primary trade-off is between softness and strength. Full annealing produces the softest possible condition. Subcritical annealing restores ductility to work-hardened parts but retains more of the original hardness compared to a full anneal.
The Confusion of "Named" Processes
You will encounter dozens of specific annealing names, such as Box, Bright, Cycle, or Spheroidizing. It is critical to understand that these are not fundamentally different processes. They are practical applications or variations of the three core thermal cycles, named for the furnace used (Box), the resulting finish (Bright), or the specific microstructure targeted (Spheroidizing).
Matching the Process to Your Goal
Your choice should always be driven by the desired end state of the material.
- If your primary focus is maximum softness, ductility, and machinability: Use full (supercritical) annealing to completely refine and reset the steel's microstructure.
- If your primary focus is restoring formability to a cold-worked part between manufacturing steps: Use subcritical (process) annealing to relieve stress and improve ductility efficiently.
- If your primary focus is achieving a specialized balance of properties for certain alloy steels: Intercritical annealing provides a path for partial transformation to meet specific requirements.
Mastering annealing means understanding how to use temperature to intentionally control the internal structure of steel.

Summary Table:
| Process | Heating Temperature Relative to Critical Points | Primary Goal | Key Outcome |
|---|---|---|---|
| Supercritical (Full) Annealing | Above Ac3 (Upper Critical) | Maximum Softness & Ductility | Completely new, refined grain structure |
| Intercritical Annealing | Between Ac1 and Ac3 | Specialized Property Balance | Partial transformation; mixed microstructure |
| Subcritical Annealing | Below Ac1 (Lower Critical) | Stress Relief & Restoring Formability | Recrystallization without phase change |
Need to Optimize Your Annealing Process?
Choosing the right annealing process is critical for achieving the precise mechanical properties your project requires. Whether you need maximum softness for machining or efficient stress relief between manufacturing steps, the right lab equipment is essential for consistent, reliable results.
KINTEK specializes in precision lab furnaces and consumables designed specifically for controlled heat treatment processes like annealing. Our equipment helps you accurately target critical temperatures, ensuring your materials achieve the desired hardness, ductility, and microstructure.
Let us help you master your heat treatment.
Contact our experts today to discuss your specific annealing application and find the ideal furnace solution for your laboratory's needs.
Visual Guide
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering Brazing Furnace
- Vacuum Heat Treat Furnace and Levitation Induction Melting Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
People Also Ask
- What materials are used in a vacuum furnace? Selecting the Right Hot Zone for Your Process
- What is the maximum temperature in a vacuum furnace? It Depends on Your Materials and Process Needs
- Can I vacuum the inside of my furnace? A Guide to Safe DIY Cleaning vs. Professional Service
- What are the uses of vacuum furnace? Achieve Unmatched Material Purity and Performance
- What does a vacuum furnace do? Achieve High-Purity Heat Treatment for Superior Components



















