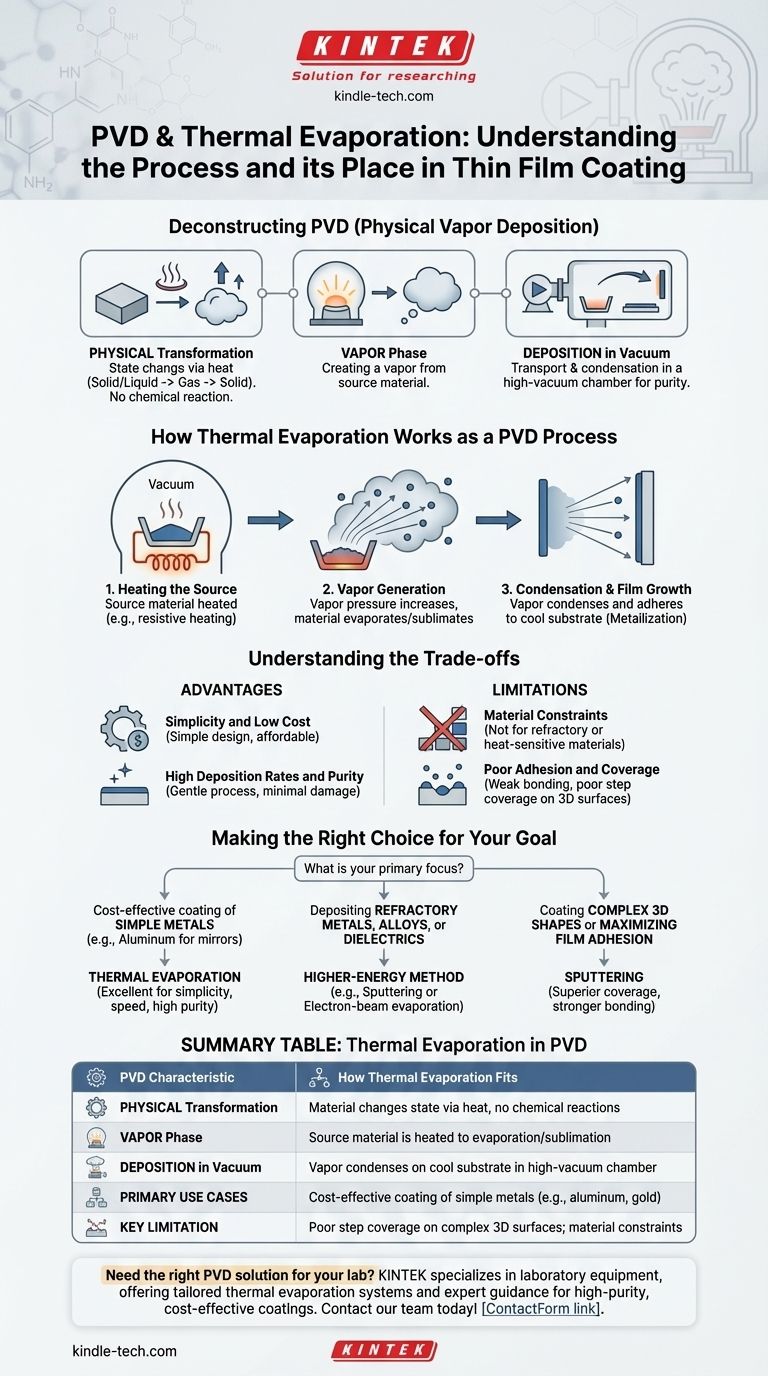
In materials science, PVD stands for Physical Vapor Deposition. It is a family of vacuum deposition techniques used to produce thin films and coatings. Thermal evaporation is not just an example of a PVD process; it is one of the most fundamental and widely used methods within this category. In this process, a source material is heated in a high vacuum until it evaporates, and the resulting vapor then travels and condenses onto a cooler substrate to form a solid film.
Physical Vapor Deposition (PVD) describes any process where a material is physically transformed into a vapor, transported through a vacuum, and condensed onto a surface as a thin film. Thermal evaporation is a classic PVD method because it accomplishes this vaporization phase using heat alone, without any chemical reactions.

Deconstructing Physical Vapor Deposition (PVD)
To understand thermal evaporation's place, you must first understand the core principles of PVD. The name itself breaks down the process into its essential components.
The "Physical" Transformation
The defining characteristic of any PVD process is that the material transfer is purely physical. The source material changes its state from solid or liquid to gas (vapor) and then back to solid, without undergoing a chemical reaction.
This contrasts with Chemical Vapor Deposition (CVD), where precursor gases react on the substrate surface to form the film.
The "Vapor" Phase
All PVD methods involve creating a vapor from the source material. The specific mechanism for creating this vapor is what distinguishes different PVD techniques from one another.
In thermal evaporation, this is achieved by heating the material. In other methods, like sputtering, it is achieved by bombarding the source with energetic ions.
The "Deposition" in a Vacuum
The entire process—vaporization, transport, and deposition—occurs inside a high-vacuum chamber. The vacuum is critical for two primary reasons.
First, it removes atmospheric gases that could react with and contaminate the vapor, ensuring the purity of the final film. Second, it creates a clear, low-pressure path for the vapor to travel from the source to the substrate without colliding with other molecules.
How Thermal Evaporation Works as a PVD Process
Thermal evaporation perfectly embodies the PVD principles. It is a line-of-sight process where evaporated atoms travel in straight lines from the source to the substrate.
Step 1: Heating the Source Material
The process begins by placing the source material, often in the form of pellets or wire, into a crucible or onto a resistive element (often called a "boat").
This source is then heated inside the vacuum chamber. Common heating methods include resistive heating (passing a current through the boat), electron-beam heating, or laser heating.
Step 2: Vapor Generation
As the material's temperature rises, its vapor pressure increases. Once it reaches a sufficiently high temperature, the material either evaporates (if it melts first) or sublimates (if it goes directly from solid to gas).
This generates a vapor cloud within the chamber. The low pressure of the vacuum allows the vapor to expand away from the source.
Step 3: Condensation and Film Growth
The vapor travels through the chamber and eventually strikes the substrate, which is intentionally kept at a much lower temperature.
Upon contact with the cool surface, the vapor rapidly cools, condenses, and adheres to the substrate, forming a solid, thin film. This process is sometimes referred to as metallization, especially when depositing metals like aluminum or gold.
Understanding the Trade-offs
While straightforward, thermal evaporation has distinct advantages and limitations compared to other PVD methods.
Advantage: Simplicity and Low Cost
Thermal evaporation systems are generally simpler in design and less expensive to operate than other PVD systems, such as those for sputtering. This makes it a highly accessible technique for many applications.
Advantage: High Deposition Rates and Purity
For many common materials, thermal evaporation can achieve high deposition rates. Because the process is "gentle" and involves low-energy particles (around 0.1 eV), it causes minimal damage to the substrate and can produce very high-purity films.
Limitation: Material Constraints
The primary limitation is that the process only works for materials that can be evaporated at temperatures that are practical to achieve in a vacuum system. Materials with extremely high melting points (refractory metals) or compounds that decompose when heated are not suitable candidates.
Limitation: Poor Adhesion and Coverage
The low kinetic energy of the evaporated particles can result in weaker film adhesion compared to higher-energy processes like sputtering. It also struggles to uniformly coat complex, three-dimensional surfaces, an issue known as poor step coverage.
Making the Right Choice for Your Goal
Selecting the correct deposition method requires matching the process characteristics to your application's needs.
- If your primary focus is cost-effective coating of simple metals (like aluminum for mirrors): Thermal evaporation is an excellent choice due to its simplicity, speed, and high material purity.
- If your primary focus is depositing refractory metals, alloys, or dielectrics: A higher-energy method like sputtering or electron-beam evaporation is often required.
- If your primary focus is coating complex 3D shapes or maximizing film adhesion: Sputtering is generally superior, as its more energetic particles provide better surface coverage and stronger bonding.
Ultimately, recognizing that thermal evaporation is a foundational PVD technique empowers you to understand its specific strengths and select it when its capabilities align with your project's goals.
Summary Table:
| PVD Characteristic | How Thermal Evaporation Fits |
|---|---|
| Physical Transformation | Material changes state via heat, no chemical reactions |
| Vapor Phase | Source material is heated to evaporation/sublimation |
| Deposition in Vacuum | Vapor condenses on cool substrate in high-vacuum chamber |
| Primary Use Cases | Cost-effective coating of simple metals (e.g., aluminum, gold) |
| Key Limitation | Poor step coverage on complex 3D surfaces; material constraints |
Need the right PVD solution for your lab's thin film coating requirements? KINTEK specializes in laboratory equipment and consumables, offering tailored thermal evaporation systems and expert guidance to help you achieve high-purity, cost-effective coatings. Contact our team today to discuss your specific application and discover how our PVD expertise can enhance your research or production process!
Visual Guide
Related Products
- Molybdenum Tungsten Tantalum Evaporation Boat for High Temperature Applications
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Hemispherical Bottom Tungsten Molybdenum Evaporation Boat
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
People Also Ask
- What is vacuum thermal evaporation? A Guide to High-Purity Thin Film Deposition
- What is the difference between sputtering and thermal evaporation? Choose the Right PVD Method for Your Thin Film
- What is the meaning of thermal evaporation? A Guide to Simple, Cost-Effective Thin Film Coating
- What is thermal effect via evaporation? A Simple Guide to Thin-Film Deposition
- What is the widely used boat made of in thermal evaporation? Choosing the Right Material for High-Purity Deposition



















