In essence, sintering is a thermal process that transforms a compacted powder into a dense, solid object by heating it to a temperature below its melting point. During this process, the individual particles of the material fuse together through atomic diffusion, dramatically increasing the part's strength and density while reducing its internal porosity.
Sintering is not about melting; it's about using heat to encourage atoms to migrate across particle boundaries. This atomic movement is the fundamental mechanism that eliminates the gaps between particles, bonding them into a coherent and strong solid mass.

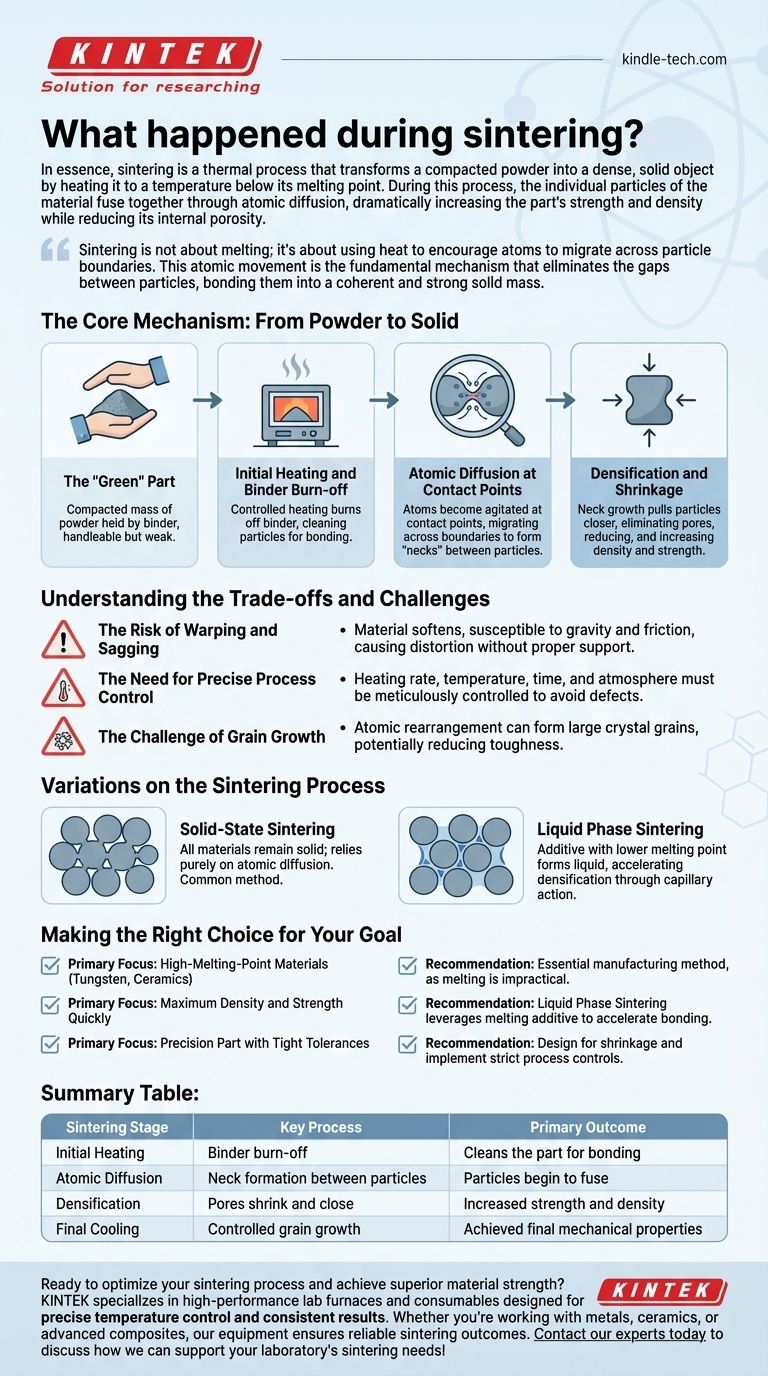
The Core Mechanism: From Powder to Solid
The journey from a loose powder to a solid component involves several distinct physical changes. Understanding these stages is key to controlling the final properties of the sintered part.
The "Green" Part
The process begins with a "green" part, which is a compacted mass of powder. This initial shape is created by pressing the powder into a die and is often held together by a temporary organic binder, giving it just enough strength to be handled.
Initial Heating and Binder Burn-off
As the green part is heated in a controlled-atmosphere furnace, the first event is the burn-off of the residual binder at relatively low temperatures. This step "cleans" the part, ensuring nothing interferes with the subsequent bonding of the material particles.
Atomic Diffusion at Contact Points
This is the heart of the sintering process. As the temperature rises significantly (but stays below the material's melting point), the atoms at the contact points between particles become highly agitated. They gain enough energy to diffuse, or move, across the boundaries from one particle to another.
This atomic migration effectively builds "necks" or bridges between adjacent particles. Driven by the reduction of surface energy, these necks grow wider, pulling the centers of the particles closer together.
Densification and Shrinkage
The collective effect of millions of particles pulling closer is a reduction in the overall volume of the part. The empty spaces, or pores, between the particles shrink and are gradually eliminated.
This results in a significant increase in the material's density and a predictable, measurable shrinkage of the component. The final dimensions and enhanced mechanical properties, such as strength and hardness, are direct outcomes of this densification.
Understanding the Trade-offs and Challenges
While powerful, sintering is a delicate process that requires precise control to avoid defects and achieve the desired outcome.
The Risk of Warping and Sagging
At sintering temperatures, the material softens long before it melts. During this phase, the part is vulnerable to gravity and friction, which can cause it to warp, sag, or distort. Proper support within the furnace is critical to maintaining dimensional accuracy.
The Need for Precise Process Control
The final properties of a sintered part are a direct function of the process variables. Factors like the heating rate, peak temperature, time at temperature, furnace atmosphere, and cooling rate must be meticulously controlled. Even small deviations can lead to insufficient density, undesirable grain growth, or internal stresses.
The Challenge of Grain Growth
While atoms are diffusing to close pores, they are also rearranging to form larger crystal grains. Excessive grain growth can sometimes be detrimental to a material's mechanical properties, such as its toughness. Controlling this is a key aspect of process optimization.
Variations on the Sintering Process
To accelerate the process or work with mixed materials, engineers can employ different types of sintering.
Solid-State Sintering
This is the fundamental process described above, where all materials involved remain in a solid form. It is the most common method and relies purely on atomic diffusion in the solid phase.
Liquid Phase Sintering
In this variation, a small amount of a secondary material with a lower melting point is mixed with the primary powder. When the furnace reaches the melting point of this additive, a liquid phase forms.
This liquid flows into the pores between the solid particles, accelerating densification through capillary action and providing a faster diffusion path. This is often used to achieve very high densities more quickly than solid-state sintering alone.
Making the Right Choice for Your Goal
Applying this knowledge depends entirely on your objective for the final component.
- If your primary focus is creating parts from very high-melting-point materials (e.g., tungsten, ceramics): Sintering is the essential manufacturing method, as melting and casting are often technically or economically unfeasible.
- If your primary focus is achieving maximum density and strength quickly: Consider liquid phase sintering, which leverages a melting additive to accelerate the bonding and densification process.
- If your primary focus is producing a precision part with tight tolerances: You must design for predictable shrinkage and implement strict process controls to prevent warping and ensure consistent final dimensions.
Ultimately, sintering is a powerful and versatile manufacturing tool that engineers materials at an atomic level to build strong parts from powder.
Summary Table:
| Sintering Stage | Key Process | Primary Outcome |
|---|---|---|
| Initial Heating | Binder burn-off | Cleans the part for bonding |
| Atomic Diffusion | Neck formation between particles | Particles begin to fuse |
| Densification | Pores shrink and close | Increased strength and density |
| Final Cooling | Controlled grain growth | Achieved final mechanical properties |
Ready to optimize your sintering process and achieve superior material strength? KINTEK specializes in high-performance lab furnaces and consumables designed for precise temperature control and consistent results. Whether you're working with metals, ceramics, or advanced composites, our equipment ensures reliable sintering outcomes. Contact our experts today to discuss how we can support your laboratory's sintering needs!
Visual Guide
Related Products
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
- Vacuum Heat Treat and Sintering Furnace with 9MPa Air Pressure
- 600T Vacuum Induction Hot Press Furnace for Heat Treat and Sintering
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Vacuum Dental Porcelain Sintering Furnace
People Also Ask
- Does sintering use diffusion? The Atomic Mechanism for Building Stronger Materials
- Why is sintering easier in the presence of a liquid phase? Unlock Faster, Lower-Temperature Densification
- Why must green bodies produced via binder jetting undergo treatment in a vacuum sintering furnace?
- Why is a high vacuum required for sintering Ti-43Al-4Nb-1Mo-0.1B? Ensure Purity & Fracture Toughness
- Why is a high vacuum environment necessary in sintering equipment for TiAl alloys? Ensure High-Purity Metal Bonding



















