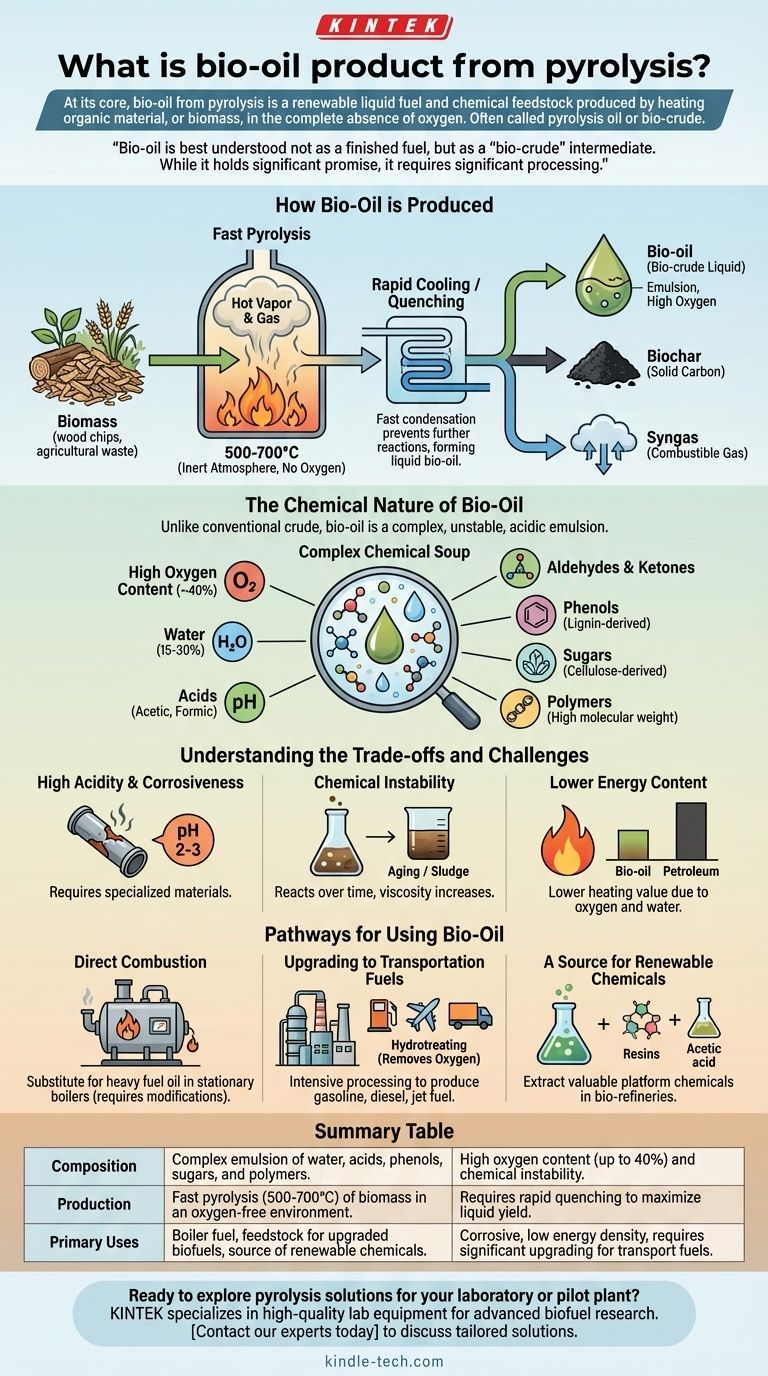
At its core, bio-oil from pyrolysis is a renewable liquid fuel and chemical feedstock produced by heating organic material, or biomass, in the complete absence of oxygen. Often called pyrolysis oil or bio-crude, this dark, viscous liquid is one of the three primary products of the pyrolysis process, alongside a solid known as biochar and a combustible gas called syngas. It represents a way to convert solid biomass, like wood or agricultural waste, into a more energy-dense and transportable liquid form.
Bio-oil is best understood not as a finished fuel, but as a "bio-crude" intermediate. While it holds significant promise as a renewable resource, its inherent instability, corrosiveness, and chemical complexity mean it requires significant processing before it can replace conventional petroleum products.

How Bio-Oil is Produced
The creation of bio-oil hinges on a thermochemical process known as pyrolysis. Understanding this process is key to understanding the nature of the oil itself.
The Pyrolysis Process
Pyrolysis is the thermal decomposition of materials at elevated temperatures in an inert atmosphere. To maximize the liquid bio-oil yield, a specific method called fast pyrolysis is typically used.
In fast pyrolysis, biomass is heated very rapidly to temperatures between 500°C and 700°C without any oxygen present. This intense heat breaks down the complex organic polymers in the biomass (like cellulose and lignin) into smaller, volatile compounds that form a hot vapor.
From Vapor to Liquid
This hot vapor is then rapidly cooled, or "quenched." This fast condensation prevents further chemical reactions and transforms the vapor into a liquid—the bio-oil.
Any non-condensable gases are collected as syngas, and the remaining solid carbon material is removed as biochar. The efficiency of this process determines the final yield of each product.
The Chemical Nature of Bio-Oil
Unlike conventional crude oil, which is a mix of hydrocarbons, bio-oil is a far more complex and challenging substance.
A Complex Chemical Soup
Bio-oil is an emulsion, not a pure substance. It contains hundreds of different organic compounds, significant amounts of water (often 15-30%), and microscopic solids.
Its most defining characteristic is its high oxygen content, which can be up to 40% by weight. This oxygen is bound within the organic molecules and is the source of many of bio-oil's unique properties and challenges.
Key Chemical Components
The liquid contains a wide range of compounds derived from the original biomass. These include:
- Acids (like acetic and formic acid)
- Aldehydes and Ketones (like formaldehyde)
- Phenols (derived from lignin)
- Sugars (derived from cellulose)
- High molecular weight polymers
This complex mixture results in a liquid that is dense, acidic, and chemically unstable.
Understanding the Trade-offs and Challenges
While bio-oil is a renewable resource, its direct application is limited by several critical factors. It is not a "drop-in" replacement for gasoline or diesel.
High Acidity and Corrosiveness
The presence of organic acids makes raw bio-oil highly acidic (pH 2-3). This makes it corrosive to common construction materials like carbon steel, requiring specialized and more expensive containers and engine components for handling and use.
Chemical Instability
Bio-oil is not stable over time. The reactive compounds within it can continue to react with each other, a process known as aging. This causes the oil's viscosity to increase, and it can eventually form solids or "sludge," making it difficult to pump or burn.
Lower Energy Content
Due to its high oxygen and water content, bio-oil has a lower heating value (energy density) than petroleum fuels. You need more bio-oil by volume to produce the same amount of energy as you would from conventional fuel oil.
Pathways for Using Bio-Oil
Given its properties, bio-oil can be used in several ways, ranging from direct use to highly complex refining.
Direct Combustion
The simplest application is to use bio-oil as a substitute for heavy fuel oil in stationary applications like industrial boilers and furnaces to generate heat or electricity. This requires modifications to the equipment to handle its corrosiveness and different combustion properties.
Upgrading to Transportation Fuels
The most valuable potential use is to upgrade it into transportation fuels. This requires intensive chemical processing, primarily hydrotreating, which uses hydrogen and catalysts to remove oxygen and stabilize the molecules. This process can theoretically convert bio-oil into fungible gasoline, diesel, and jet fuel.
A Source for Renewable Chemicals
Bio-oil can also be seen as a liquid mine for "green" chemicals. Through complex separation and refining processes, valuable platform chemicals like phenols (for resins) and acetic acid can be extracted, providing a renewable alternative to petroleum-based chemical production.
Making the Right Choice for Your Goal
Viewing bio-oil correctly is crucial for any project involving pyrolysis. It is a stepping stone, not a final destination.
- If your primary focus is decentralized energy: See bio-oil as a way to convert local biomass into a liquid boiler fuel, but plan for the necessary equipment modifications and handling protocols.
- If your primary focus is creating advanced biofuels: Treat bio-oil as a crude intermediate that requires a significant and technically complex upgrading facility to become a viable drop-in fuel.
- If your primary focus is a sustainable circular economy: View bio-oil as a potential feedstock for a new generation of bio-refineries capable of producing both fuels and renewable chemicals.
Ultimately, bio-oil is a critical but challenging link in the chain of converting biomass into modern energy and materials.
Summary Table:
| Property | Description | Key Challenge |
|---|---|---|
| Composition | Complex emulsion of water, acids, phenols, sugars, and polymers. | High oxygen content (up to 40%) and chemical instability. |
| Production | Fast pyrolysis (500-700°C) of biomass in an oxygen-free environment. | Requires rapid quenching to maximize liquid yield. |
| Primary Uses | Boiler fuel, feedstock for upgraded biofuels, source of renewable chemicals. | Corrosive, low energy density, requires significant upgrading for transport fuels. |
Ready to explore pyrolysis solutions for your laboratory or pilot plant?
KINTEK specializes in high-quality lab equipment and consumables for advanced biofuel and biochemical research. Whether you are developing pyrolysis processes, analyzing bio-oil properties, or upgrading feedstocks, our reliable equipment helps you achieve accurate and reproducible results.
Contact our experts today to discuss how we can support your renewable energy and chemical production goals with tailored solutions for your laboratory needs.
Visual Guide
Related Products
- Electric Rotary Kiln Pyrolysis Furnace Plant Machine Calciner Small Rotary Kiln Rotating Furnace
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Heating Pyrolysis Plant
- Customizable PEM Electrolysis Cells for Diverse Research Applications
- FS Electrochemical Hydrogen Fuel Cells for Diverse Applications
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
People Also Ask
- What is the purpose of a calciner? Boost Efficiency in High-Temperature Processing
- What are the products of pyrolysis of wood? A Guide to Biochar, Bio-oil, and Syngas Yields
- What are the zones in rotary kiln in cement production? Master the Core Process for High-Quality Clinker
- What is the difference between calcining and roasting? A Guide to High-Temperature Processing
- What are the types of pyrolysis reactors used in industry? Choose the Right Technology for Your Product
















