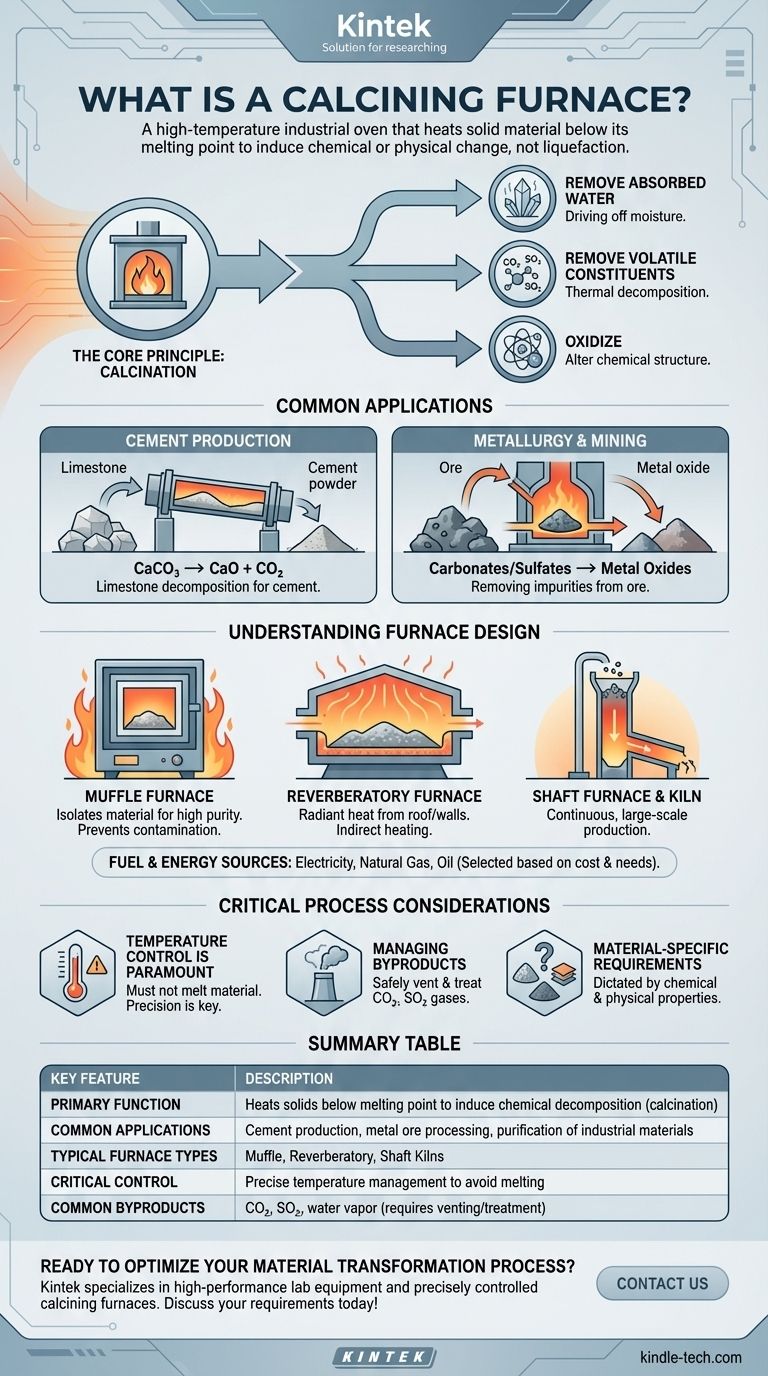
At its core, a calcining furnace is a high-temperature industrial oven that heats a solid material to a precise temperature just below its melting point. This controlled heating is not meant to liquefy the material, but rather to induce a chemical or physical change, such as thermal decomposition or the removal of a volatile fraction.
The critical purpose of a calcining furnace is not simply to heat a substance, but to fundamentally alter it. By carefully applying heat without melting, these furnaces drive off moisture, break down chemical compounds, and purify materials for industrial use.

The Core Principle of Calcination
What is Calcination?
Calcination is a thermal treatment process applied to ores and other solid materials. The defining characteristic is heating the substance to a high temperature, but remaining below its fusion or melting point.
This triggers "thermal decomposition," where heat alone breaks down the material's chemical structure.
Key Objectives of the Process
The goal of calcination is typically one of three outcomes:
- Removing absorbed water (driving off "free" or crystal-bound moisture).
- Removing volatile constituents like carbon dioxide (CO₂) or sulfur dioxide (SO₂).
- Oxidizing a portion or the entirety of the substance.
Common Applications Across Industries
Cement Production
The most widespread use of calcination is in manufacturing cement. In this process, a calcining furnace or kiln heats limestone (calcium carbonate, CaCO₃).
The heat decomposes the limestone into calcium oxide (CaO), also known as quicklime, and releases carbon dioxide gas. This calcium oxide is the primary ingredient in cement.
Metallurgy and Mining
Calcination is a fundamental step in extracting metals from certain ores. For instance, ores mined as carbonates or sulfates are heated in a furnace.
This process removes the carbonate or sulfate group, leaving behind a metal oxide which can then be more easily reduced to the pure metal.
Understanding the Furnace Design
Common Furnace Configurations
While designs vary based on the specific material and desired output, most calcining furnaces fall into one of three categories:
- Muffle Furnaces: These isolate the material from the fuel and combustion byproducts, ideal for processes requiring high purity.
- Reverberatory Furnaces: Heat is radiated from the roof and walls onto the material, allowing for indirect heating.
- Shaft Furnaces & Kilns: Material is fed into the top of a vertical chamber and heated as it moves down, often used for continuous, large-scale production.
Fuel and Energy Sources
The energy source for a calcining furnace is selected based on application requirements, cost, and availability. Common fuels include electricity, natural gas, and oil.
Critical Process Considerations
Temperature Control is Paramount
The entire process hinges on precise temperature management. Overheating the material past its melting point would defeat the purpose of calcination, resulting in a fused, unusable mass instead of a decomposed powder or solid.
Managing Byproducts
The decomposition process releases significant volumes of gas, such as CO₂ or SO₂. A properly designed furnace system must include provisions for safely venting and, where necessary, treating these gaseous byproducts.
Material-Specific Requirements
The exact temperature and duration of the calcination process are dictated entirely by the chemical and physical properties of the material being treated. What works for limestone will not work for a metallic ore.
Making the Right Choice for Your Goal
Understanding the end goal is key to selecting the correct approach.
- If your primary focus is bulk commodity production (e.g., cement): A continuous-feed system like a shaft kiln is designed for the high throughput and efficiency you require.
- If your primary focus is high-purity material processing: A muffle furnace is the ideal choice as it prevents contamination from fuel combustion.
- If your primary focus is extracting a specific metal from ore: The furnace type and temperature profile must be precisely matched to the ore's chemical composition.
Ultimately, mastering calcination is about using controlled heat to transform a material's chemistry, not just its temperature.
Summary Table:
| Key Feature | Description |
|---|---|
| Primary Function | Heats solids below melting point to induce chemical decomposition (calcination) |
| Common Applications | Cement production, metal ore processing, purification of industrial materials |
| Typical Furnace Types | Muffle, Reverberatory, Shaft Kilns |
| Critical Control Parameter | Precise temperature management to avoid melting |
| Common Byproducts | CO₂, SO₂, water vapor (requires venting/treatment) |
Ready to optimize your material transformation process? KINTEK specializes in high-performance lab equipment, including precisely controlled calcining furnaces for cement, metallurgy, and high-purity applications. Our experts will help you select the ideal furnace configuration—whether you need contamination-free muffle furnaces for purity or continuous kilns for bulk production. Contact us today to discuss your specific calcination requirements and enhance your operational efficiency!
Visual Guide
Related Products
- Electric Rotary Kiln Pyrolysis Furnace Plant Machine Calciner Small Rotary Kiln Rotating Furnace
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Heating Pyrolysis Plant
- Rotary Tube Furnace Split Multi Heating Zone Rotating Tube Furnace
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
People Also Ask
- What is the difference between calcining and roasting? A Guide to High-Temperature Processing
- What is the purpose of a calciner? Boost Efficiency in High-Temperature Processing
- What biomass is used in pyrolysis? Selecting the Optimal Feedstock for Your Goals
- What is the principle of rotary kiln? Mastering Continuous Thermal Processing
- What are the industrial applications of pyrolysis? Transform Waste into Energy and Valuable Products



















