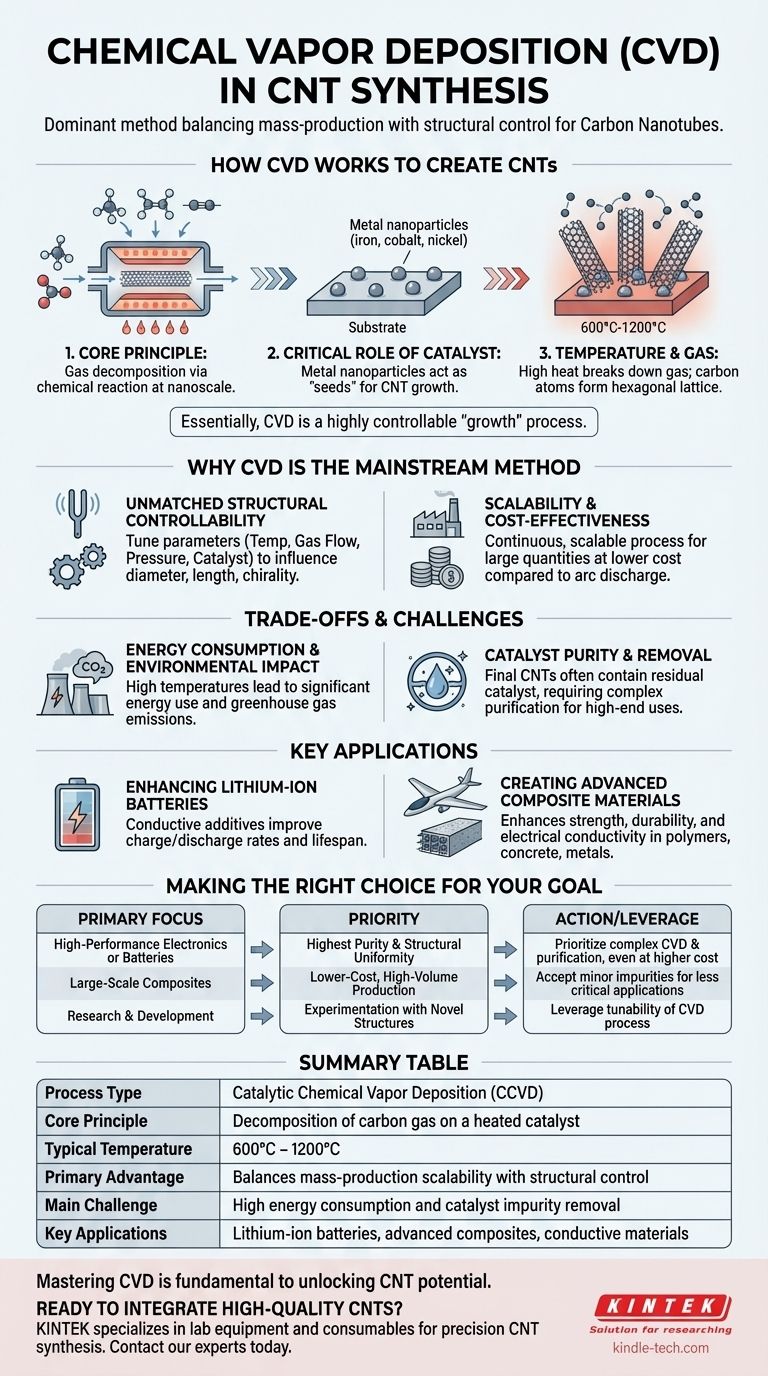
In the context of carbon nanotubes (CNTs), Chemical Vapor Deposition (CVD) is the dominant industrial method used to synthesize them. It is a process where a substrate, prepared with a layer of catalyst nanoparticles, is heated in a furnace while a carbon-containing gas is introduced. The high temperature causes the gas to decompose, and the carbon atoms then assemble into nanotube structures on the surface of the catalyst particles.
At its core, CVD is not just a manufacturing technique; it is a highly controllable "growth" process. Its widespread adoption stems from its unique ability to balance mass-production scalability with precise control over the final nanotube structure, a combination that older methods could not achieve.

How CVD Fundamentally Works to Create CNTs
To understand why CVD is the standard, you must first grasp its core principles. The process is a carefully orchestrated chemical reaction occurring at the nanoscale.
The Core Principle: A Reaction in Gas Form
The entire process is defined by three characteristics. First, a chemical reaction (or thermal decomposition) must occur. Second, the carbon atoms that form the nanotube film come from an external source—the gas. Third, these source materials must be in a gaseous state to participate in the reaction.
The Critical Role of the Catalyst
This process is more accurately called Catalytic CVD (CCVD) because a catalyst is non-negotiable. Tiny metal nanoparticles (often iron, cobalt, or nickel) are deposited onto a substrate. These particles act as the "seeds" from which the carbon nanotubes grow, dictating their diameter and structure.
The Importance of Temperature and Gas
The reaction chamber is heated to high temperatures, typically between 600°C and 1200°C. This extreme heat provides the energy needed to break down a hydrocarbon gas (like methane, ethylene, or acetylene) that is flowed into the chamber. The carbon atoms are freed and diffuse onto the catalyst, where they assemble into the hexagonal lattice of a nanotube.
Why CVD is the Mainstream Method
Older methods like arc discharge and laser ablation can produce high-quality CNTs, but they fail to match the scalability and control offered by CVD.
Unmatched Structural Controllability
CVD gives manufacturers significant control over the outcome. By carefully tuning the parameters—such as temperature, gas flow rate, pressure, and the choice of catalyst—it is possible to influence the nanotubes' diameter, length, and even chirality. This is critical for high-tech applications where specific properties are required.
Scalability and Cost-Effectiveness
Compared to the extreme conditions of arc discharge or laser ablation, CVD is a more continuous and scalable process. It allows for the production of large quantities of CNTs at a lower cost, making them commercially viable for use as additives in materials like batteries and composites.
Understanding the Trade-offs and Challenges
While powerful, the CVD process is not without its challenges. True expertise requires acknowledging its limitations.
Energy Consumption and Environmental Impact
The high temperatures required for CVD mean the process is energy-intensive. The synthesis process is the primary source of potential ecotoxicity in the CNT life cycle, driven by material consumption, energy use, and the emission of greenhouse gases.
Catalyst Purity and Removal
A significant challenge is that the final CNT product is often contaminated with residual catalyst particles. For high-performance applications like electronics and batteries, these metallic impurities must be removed through complex and costly purification steps.
Where CVD-Grown CNTs Are Used
The ability to produce CNTs at scale via CVD has unlocked their use in a wide range of fields, especially in green technologies.
Enhancing Lithium-Ion Batteries
CNTs are primarily used as conductive additives in battery cathodes and anodes. Their exceptional conductivity improves the battery's charge/discharge rates and overall lifespan.
Creating Advanced Composite Materials
When added to polymers, concrete, or metals, CNTs can dramatically enhance strength, durability, and electrical conductivity. This has led to applications in everything from lightweight aerospace components to conductive plastics and stronger concrete.
Making the Right Choice for Your Goal
Understanding the fundamentals of CVD allows you to align the synthesis method with your specific technical objective.
- If your primary focus is high-performance electronics or batteries: Your priority should be CVD processes that yield the highest purity and structural uniformity, even if it requires more complex catalyst and purification steps.
- If your primary focus is large-scale composites (like concrete or polymers): You can prioritize lower-cost, high-volume CVD methods where minor impurities from the catalyst are less critical to the final application's performance.
- If your primary focus is research and development: Leverage the inherent tunability of the CVD process to experiment with different catalysts, gases, and temperatures to create novel nanotube structures with unique properties.
Mastering the levers of the CVD process is fundamental to unlocking the transformative potential of carbon nanotubes in any application.
Summary Table:
| Aspect | Key Detail |
|---|---|
| Process Type | Catalytic Chemical Vapor Deposition (CCVD) |
| Core Principle | Decomposition of carbon gas on a heated catalyst |
| Typical Temperature | 600°C - 1200°C |
| Primary Advantage | Balances mass-production scalability with structural control |
| Main Challenge | High energy consumption and catalyst impurity removal |
| Key Applications | Lithium-ion batteries, advanced composites, conductive materials |
Ready to integrate high-quality CNTs into your R&D or production? The right lab equipment is crucial for optimizing your CVD process, whether your goal is high-purity electronics or large-scale composites. KINTEK specializes in lab equipment and consumables, serving laboratory needs with precision furnaces, gas handling systems, and catalysts essential for controlled CNT growth. Contact our experts today to discuss how our solutions can help you achieve your specific CNT synthesis goals.
Visual Guide
Related Products
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
- Vacuum Hot Press Furnace Heated Vacuum Press Machine Tube Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 600T Vacuum Induction Hot Press Furnace for Heat Treat and Sintering
People Also Ask
- What is the floating catalyst method? A Guide to High-Yield CNT Production
- What is a CVD tube furnace? A Complete Guide to Thin-Film Deposition
- How high of temperature do carbon nanotubes in air have the ability to sustain? Understanding the Oxidation Limit
- What are the main advantages of Chemical Vapor Deposition (CVD)? Achieve Precision Coating for Complex Geometries
- How does chirality affect carbon nanotubes? It Determines If They Are Metal or Semiconductor



















