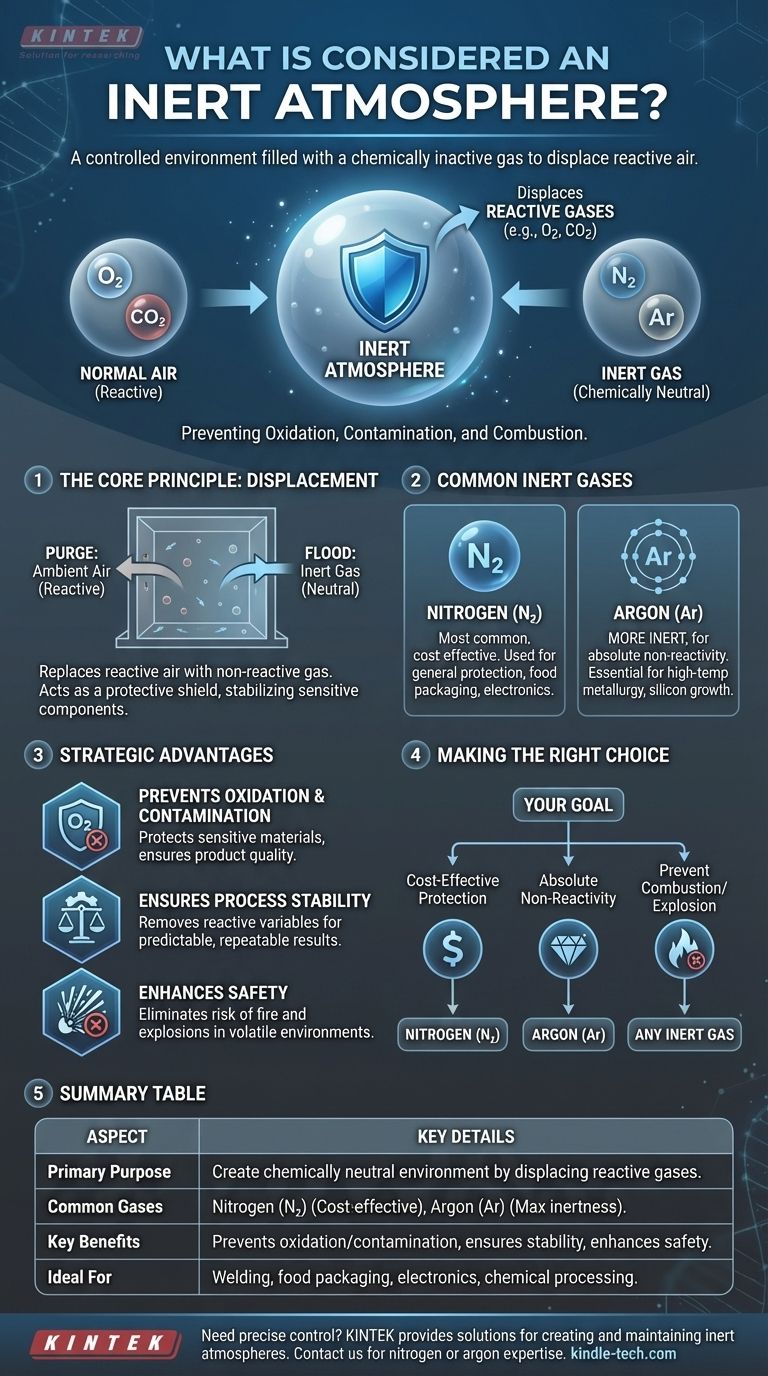
In short, an inert atmosphere is a controlled environment filled with a chemically inactive gas. This special atmosphere is engineered to displace reactive gases found in normal air, like oxygen and carbon dioxide, thereby preventing unwanted chemical reactions such as oxidation or contamination during a sensitive process.
The fundamental purpose of an inert atmosphere is to create a chemically neutral space. By replacing reactive air with a non-reactive gas like nitrogen or argon, you can protect materials and processes from degradation, contamination, or combustion.

The Core Principle: Preventing Unwanted Reactions
To understand why inert atmospheres are critical in so many technical fields, we must first understand the problem they solve: the reactivity of air.
The Problem with a Normal Atmosphere
The air we breathe is approximately 21% oxygen. While essential for life, oxygen is a highly reactive element that readily combines with other substances in a process called oxidation.
This process can be destructive, causing metals to rust, sensitive chemicals to degrade, and flammable materials to combust. Similarly, other gases in the air, like carbon dioxide, can also interfere with precise chemical operations.
How an Inert Atmosphere Solves This
An inert atmosphere works by simple displacement. A chamber or workspace is purged of ambient air and flooded with a gas that has a very low chemical reactivity.
Because the inert gas will not easily react with other materials, it acts as a protective shield. This environment stabilizes sensitive components and allows processes like welding, 3D printing with metal powders, or chemical manufacturing to occur without the risk of unwanted side reactions.
Common Gases and Their Applications
The choice of gas depends on the specific application, the sensitivity of the materials involved, and the required level of purity.
Nitrogen (N₂)
Nitrogen is the most common and cost-effective gas used for creating inert atmospheres. It is suitable for a wide range of applications, from food packaging (to prevent spoilage) to electronics manufacturing.
Argon (Ar)
Argon is more inert than nitrogen and is used when absolute non-reactivity is required. It is essential for high-temperature metallurgical processes, such as welding titanium or growing silicon crystals, where even the slightest reaction with nitrogen would be detrimental.
Strategic Advantages of an Inert Environment
Employing an inert atmosphere provides several distinct operational and safety benefits that are critical for high-precision work.
Preventing Oxidation and Contamination
This is the primary advantage. By eliminating oxygen, an inert atmosphere protects sensitive materials from degradation, ensuring the final product meets its required chemical and structural specifications.
Ensuring Process Stability
Chemical reactions can be unpredictable, especially under changing conditions like high temperatures. An inert atmosphere removes reactive variables, leading to a more stable and repeatable process with predictable outcomes.
Enhancing Safety
Many industrial processes involve fine powders or volatile chemicals that can be flammable or explosive in the presence of oxygen. By displacing oxygen, an inert atmosphere effectively eliminates the risk of fire and explosions.
Making the Right Choice for Your Goal
Selecting the appropriate inerting strategy depends entirely on the sensitivity of your materials and the goals of your process.
- If your primary focus is cost-effective protection for general applications: Nitrogen is almost always the correct choice due to its low cost and wide availability.
- If your primary focus is absolute non-reactivity for sensitive materials: Argon is the required standard for high-temperature metallurgy or processes where nitrogen could form unwanted nitrides.
- If your primary focus is preventing combustion or explosion: Any inert gas that effectively displaces oxygen will provide a safe operating environment.
Ultimately, mastering the use of an inert atmosphere gives you precise control over the chemical environment, ensuring process integrity and safety.
Summary Table:
| Aspect | Key Details |
|---|---|
| Primary Purpose | To create a chemically neutral environment by displacing reactive gases (e.g., oxygen). |
| Common Gases | Nitrogen (N₂) for cost-effectiveness; Argon (Ar) for maximum inertness. |
| Key Benefits | Prevents oxidation/contamination, ensures process stability, enhances safety. |
| Ideal For | Welding, food packaging, electronics manufacturing, chemical processing. |
Need precise control over your process environment? KINTEK specializes in lab equipment and consumables, providing solutions for creating and maintaining inert atmospheres. Whether you require nitrogen for general applications or argon for highly sensitive materials, our expertise ensures your processes are protected from oxidation, contamination, and combustion risks. Contact our experts today to discuss how we can enhance the safety and integrity of your laboratory work.
Visual Guide
Related Products
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What role does a high-temperature hydrogen atmosphere furnace play in the heat treatment of tungsten plates?
- What is the function of a high-temperature atmosphere box furnace in boriding and chromizing? Optimize Surface Engineering
- What is an atmosphere furnace? A Guide to Controlled Environment Heating
- What is the key role of a high-temperature atmosphere furnace in assessing oxidation? Evaluate Superalloy Durability
- What is a high temperature inert atmosphere furnace? Control Your Heat Treatment Process
- What is the function of an atmosphere controlled high-temperature furnace in biochar production? Master Pyrolysis Control
- What is the role of shielding gases in brazing? Optimize Your Brazing Process for Superior Results
- What is the protective atmosphere in heat treatment? Master the Key to Precision Metallurgy



















