In essence, Chemical Vapor Deposition (CVD) for graphene is a precision-engineering process used to grow a single, continuous layer of carbon atoms on a substrate. It involves introducing a carbon-containing gas, such as methane, into a high-temperature chamber where it decomposes. A metal foil, typically copper, acts as a catalyst, providing a surface where the carbon atoms can arrange themselves into the hexagonal lattice structure of graphene.
CVD is not simply a recipe for making graphene; it is a scalable manufacturing technique. Its core advantage lies in its ability to assemble high-quality, single-layer graphene from the bottom up over large surface areas, making it the leading method for industrial and electronic applications.

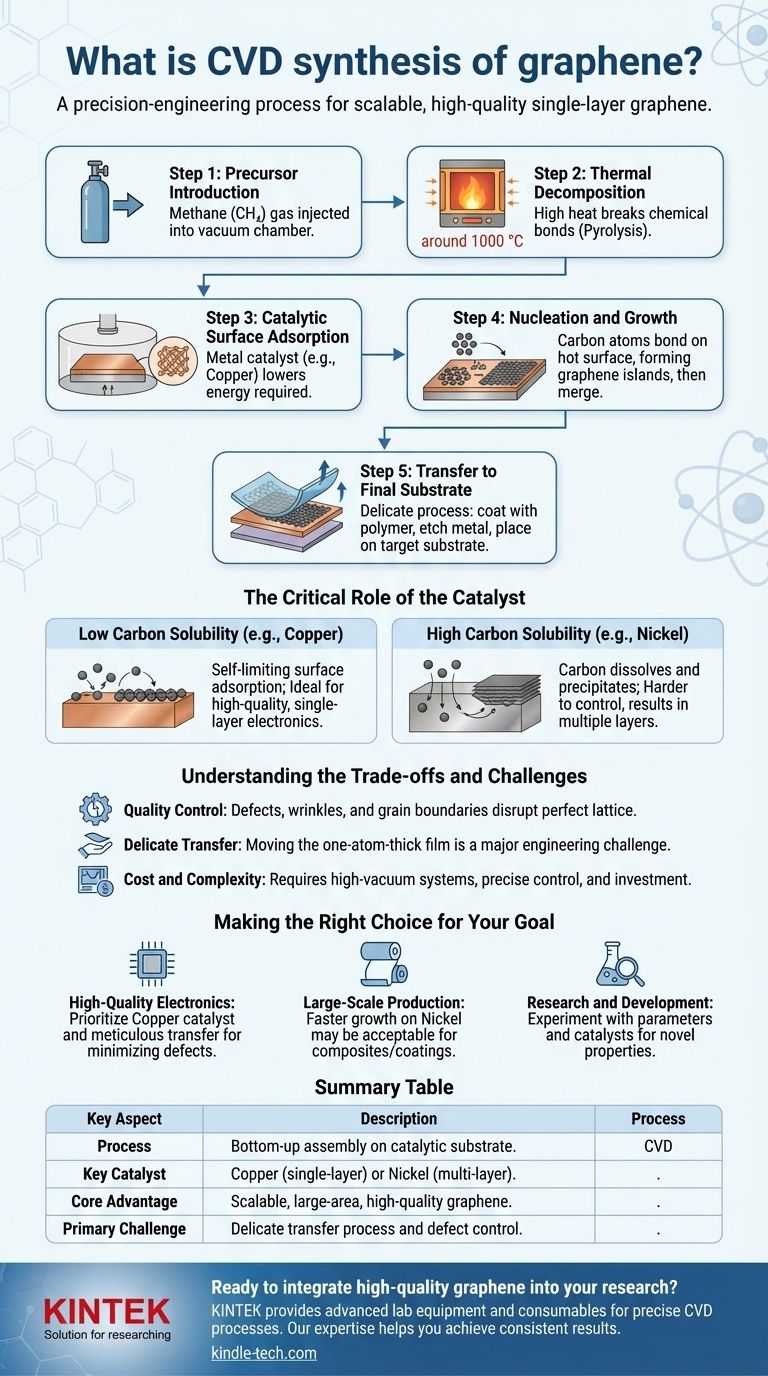
The Core Mechanism: A Step-by-Step Breakdown
To understand CVD, it is best to view it as a controlled, sequential process of atomic assembly. Each step is critical to the quality of the final graphene film.
Step 1: Precursor Introduction
A volatile, carbon-based precursor gas, most commonly methane (CH₄), is injected into a sealed chamber under vacuum conditions.
Step 2: Thermal Decomposition
The chamber is heated to extremely high temperatures, typically around 1000 °C. This intense heat provides the energy to break the chemical bonds in the precursor gas, a process known as pyrolysis, which frees individual carbon atoms.
Step 3: Catalytic Surface Adsorption
Inside the chamber is a metal substrate, such as copper (Cu) foil. This metal is not just a surface to grow on; it acts as a catalyst, dramatically lowering the energy required for the decomposition reaction to occur.
Step 4: Nucleation and Growth
The free carbon atoms diffuse across the hot metal surface. They begin to bond with each other, forming small islands or "nuclei" of graphene. As more carbon atoms attach to the edges of these islands, they grow and eventually merge to form a continuous, single-atom-thick sheet covering the substrate.
Step 5: Transfer to Final Substrate
Because graphene is most useful on insulating substrates like silicon wafers or plastics, the newly formed film must be transferred. This delicate process typically involves coating the graphene with a polymer support, etching away the metal catalyst, and carefully placing the graphene-polymer sheet onto the target substrate.
The Critical Role of the Catalyst
The choice of metal catalyst is the most significant factor determining the final properties of the graphene. The interaction is governed by the metal's carbon solubility.
Low Carbon Solubility (e.g., Copper)
Copper has very low carbon solubility. This means carbon atoms do not dissolve into the bulk metal. Instead, the process is limited to the surface. This surface-adsorption mechanism is self-limiting, typically stopping once a complete monolayer of graphene has formed, making copper the ideal catalyst for high-quality, single-layer electronic applications.
High Carbon Solubility (e.g., Nickel)
Nickel has high carbon solubility. At high temperatures, carbon atoms first dissolve into the nickel foil. As the foil cools, the solubility drops, and the carbon "precipitates" back out onto the surface to form graphene. This diffusion and segregation mechanism is harder to control and can easily result in the formation of multiple, inconsistent graphene layers.
Understanding the Trade-offs and Challenges
While CVD is the most promising method for scalable graphene production, it is not without its complexities. Acknowledging these challenges is key to successful implementation.
Quality Control is Paramount
The "perfect" hexagonal lattice of graphene can be disrupted by defects, wrinkles, and grain boundaries where different growth islands meet. These imperfections can degrade the material's exceptional electronic and mechanical properties.
The Transfer Process is Delicate
Moving a one-atom-thick film without tearing, wrinkling, or contaminating it is a significant engineering challenge. The transfer step is often the source of most defects found in the final product.
Cost and Complexity
Although described as relatively inexpensive for large-area production, CVD requires significant capital investment. It depends on high-vacuum systems, precise gas flow controllers, and high-temperature furnaces, all of which demand expertise to operate consistently.
Making the Right Choice for Your Goal
Your choice of CVD parameters is dictated entirely by your end-use application.
- If your primary focus is high-quality electronics: Prioritize a copper catalyst for its self-limiting monolayer growth and meticulously control the transfer process to minimize defects.
- If your primary focus is large-scale production for composites or coatings: A faster growth process on a catalyst like nickel may be acceptable, even if it produces minor defects or multiple layers where ultimate electronic performance is not the goal.
- If your primary focus is research and development: Experiment with different precursors, temperatures, and catalysts to tune the specific properties of the graphene film for novel applications.
Ultimately, mastering the CVD process is about controlling atomic-scale assembly to unlock the remarkable potential of graphene.
Summary Table:
| Key Aspect | Description |
|---|---|
| Process | Bottom-up assembly of carbon atoms on a catalytic substrate. |
| Key Catalyst | Copper (for single-layer) or Nickel (for multi-layer). |
| Core Advantage | Scalable production of large-area, high-quality graphene. |
| Primary Challenge | Delicate transfer process and defect control. |
Ready to integrate high-quality graphene into your research or product development?
KINTEK specializes in providing the advanced lab equipment and consumables necessary for precise CVD processes. Whether you are developing next-generation electronics or advanced composite materials, our expertise and reliable solutions help you achieve consistent, high-quality results.
Contact our experts today to discuss how we can support your specific graphene synthesis goals.
Visual Guide
Related Products
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
People Also Ask
- What is the process of deposition in a wafer? A Guide to CVD and PVD Methods
- What is the deposition rate of MOCVD? Master the Key to High-Quality Thin Film Growth
- What is the process of vacuum vapor deposition? Mastering CVD and PVD Thin-Film Coating
- What substance is used to make lab-grown diamonds? Pure Carbon, Identical to Natural Diamonds
- What are the different types of CVD deposition? Choose the Right Method for Your Thin Film Needs
- What is the chemical vapor deposition process for thin film? Grow Superior, Conformal Coatings
- What is the main difference between ALD and CVD? Precision vs. Speed in Thin Film Deposition
- What are the techniques of chemical vapor deposition? A Guide to Thermal, Plasma, and LPCVD Methods



















