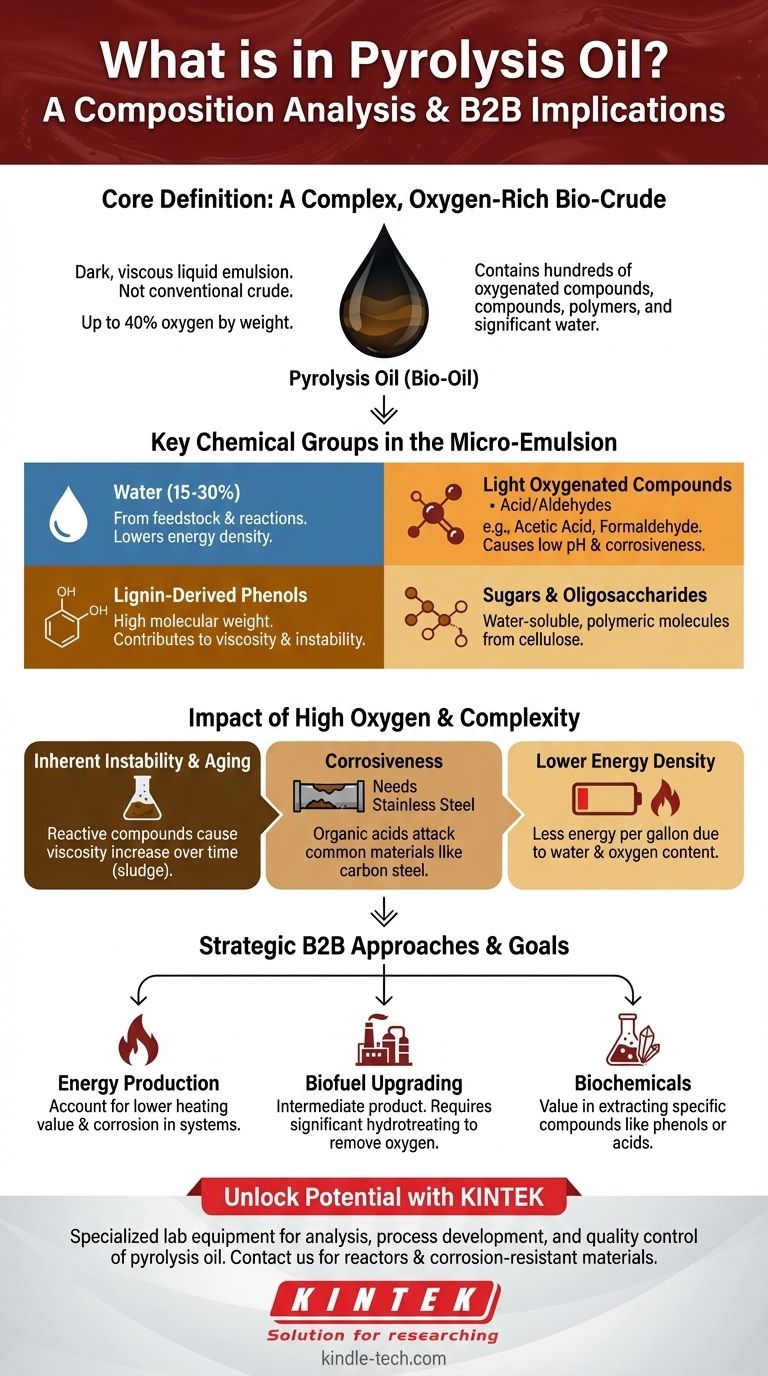
In essence, pyrolysis oil is a complex, oxygen-rich liquid emulsion. Often called bio-oil or bio-crude, it is a dark, viscous fluid composed of hundreds of different oxygenated organic compounds, polymers, and a significant amount of water. Its composition is fundamentally different from conventional crude oil due to its high oxygen content, which can be as much as 40% by weight.
The defining characteristic of pyrolysis oil is its chemical complexity and high oxygen content. This mixture of acids, aldehydes, phenols, and water makes it highly corrosive and unstable, posing significant challenges for its direct use as a fuel without further processing.

The Core Chemical Groups in Pyrolysis Oil
Pyrolysis oil is not a single substance but a micro-emulsion containing a vast array of molecules derived from the thermal decomposition of biomass. We can categorize its contents into a few key groups.
Water
A significant portion of pyrolysis oil is water, often ranging from 15-30%. This water comes from the original moisture in the biomass feedstock and as a product of the chemical reactions during pyrolysis.
Light Oxygenated Compounds
This group includes a wide variety of low molecular weight chemicals. These are the primary contributors to the oil's low pH (high acidity) and distinct acrid smell.
Common examples include acetic acid and formaldehyde, as well as other aldehydes, ketones, and furans.
Lignin-Derived Phenols
When the lignin in biomass breaks down, it forms a range of phenolic compounds. These are higher molecular weight molecules that contribute to the oil's viscosity and instability over time.
Sugars and Oligosaccharides
Derived from the cellulose and hemicellulose in the feedstock, these are water-soluble carbohydrate-based compounds. They exist as larger, sometimes polymeric, molecules within the oil.
Why This Composition Matters
Understanding what is in pyrolysis oil is critical because its components dictate its behavior, limitations, and potential uses. The composition presents a double-edged sword: it holds potential for valuable chemicals but creates problems for use as a simple fuel.
The Impact of High Oxygen Content
The most critical differentiator from petroleum is the high oxygen content. While petroleum is almost entirely hydrocarbons (hydrogen and carbon), pyrolysis oil's oxygen content of up to 40% is the source of its main challenges.
This oxygen is bound within acidic, aldehydic, and phenolic functional groups, making the oil inherently reactive and unstable.
Inherent Instability and Aging
The reactive compounds within the oil can continue to react with each other during storage. This process, known as aging, causes the oil to increase in viscosity, eventually forming sludge and solid polymers that can clog equipment.
Understanding the Trade-offs
The unique composition of pyrolysis oil creates a distinct set of challenges that must be managed. It is not a "drop-in" replacement for conventional crude oil.
The Challenge of Corrosiveness
The presence of organic acids, primarily acetic acid, makes raw pyrolysis oil highly corrosive to common construction materials like carbon steel. This necessitates the use of more expensive stainless steel for storage tanks, pumps, and piping.
Lower Energy Density
Because a large fraction of the oil's weight is composed of oxygen and water (which do not combust), its heating value is significantly lower than that of fossil fuels. You get less energy per gallon.
The Need for Upgrading
Due to its corrosiveness, instability, and low energy density, pyrolysis oil almost always requires further processing, known as upgrading. This typically involves catalytic reactions with hydrogen (hydrotreating) to remove oxygen and stabilize the molecules, making it more like conventional crude.
Making the Right Choice for Your Goal
Your approach to pyrolysis oil depends entirely on your end goal, as its complex composition can be either a challenge to overcome or a resource to exploit.
- If your primary focus is energy production: You must account for its lower heating value and corrosive nature when designing combustion or storage systems.
- If your primary focus is producing biofuels: Recognize that pyrolysis oil is an intermediate product, not a final fuel, requiring significant upgrading to remove oxygen and improve stability.
- If your primary focus is creating biochemicals: The value lies in developing separation technologies to extract specific high-value compounds like phenols or acids from the complex mixture.
Understanding the intricate and oxygenated nature of pyrolysis oil is the first step in unlocking its potential as a renewable resource.
Summary Table:
| Component | Typical Content | Key Characteristics |
|---|---|---|
| Water | 15-30% | From feedstock moisture and reactions; lowers energy density. |
| Light Oxygenated Compounds | Varies | Includes acetic acid, formaldehyde; causes low pH and corrosiveness. |
| Lignin-Derived Phenols | Varies | High molecular weight; contributes to viscosity and instability. |
| Sugars and Oligosaccharides | Varies | Derived from cellulose/hemicellulose; water-soluble polymers. |
Unlock the Potential of Your Biomass with KINTEK
Understanding the complex composition of pyrolysis oil is just the first step. Whether your goal is energy production, biofuel upgrading, or chemical extraction, having the right laboratory equipment is crucial for analysis, process development, and quality control.
KINTEK specializes in providing high-quality lab equipment and consumables tailored to the needs of researchers and engineers working in renewable energy and bio-based chemicals. From reactors and analyzers to corrosion-resistant materials, we supply the tools you need to tackle the challenges of pyrolysis oil.
Ready to advance your bio-oil research or process? Contact our experts today to discuss how KINTEK can support your laboratory's specific requirements and help you turn biomass into valuable products.
Visual Guide
Related Products
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Heating Pyrolysis Plant
- Laboratory Sterilizer Lab Autoclave Pulsating Vacuum Desktop Steam Sterilizer
- Advanced Engineering Fine Ceramics Boron Nitride (BN) Ceramic Parts
- Boron Nitride (BN) Ceramic Tube
- Customizable PEM Electrolysis Cells for Diverse Research Applications
People Also Ask
- What does a rotary vacuum evaporator do? Gently Remove Solvents for Precise Sample Concentration
- How does the centrifuge process work? Unlock Rapid Separation for Your Lab
- What are the heating rate requirements for a fast pyrolysis furnace? Achieve Max Bio-Oil Yield with Rapid Thermal Shock
- What are the advantages disadvantages and uses of sheet metal? The Ultimate Guide to Material Selection
- Where is cold-rolled steel used? Applications from Automotive to Appliances
- What is a target in a sputtering process? The Source of Your Thin Film Coating
- How much does it cost to make a lab grown diamond? The True Price of High-Tech Gem Creation
- What is a reactive sputtering reaction? Synthesize Advanced Thin Films with Precision














