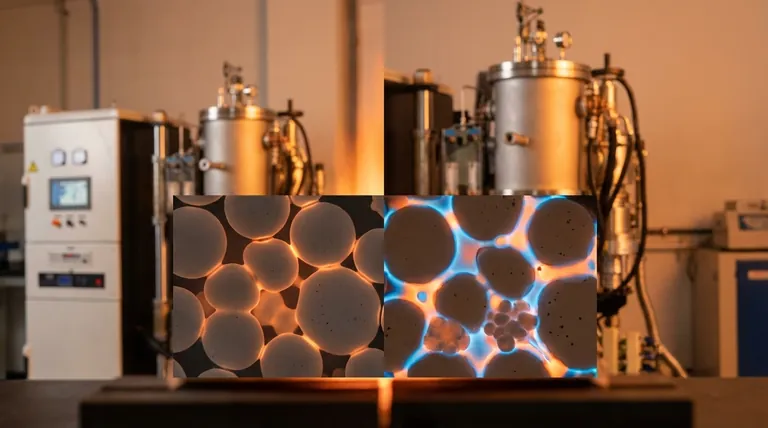
At its core, liquid phase sintering uses a small amount of a molten additive to dramatically accelerate the bonding of powder particles, while solid-state sintering achieves this bonding purely through atomic diffusion between solid particles. The liquid acts as a transport medium and binding agent, allowing for densification at lower temperatures and in less time than is possible with solid-state methods alone.
The fundamental choice between these two processes is not about which is superior, but which mechanism is required to overcome a material's inherent resistance to densification. Liquid phase sintering provides an engineered shortcut for difficult materials, whereas solid-state sintering offers a path to chemical purity.
The Foundation: What is Sintering?
The Goal: Bonding Particles into a Solid
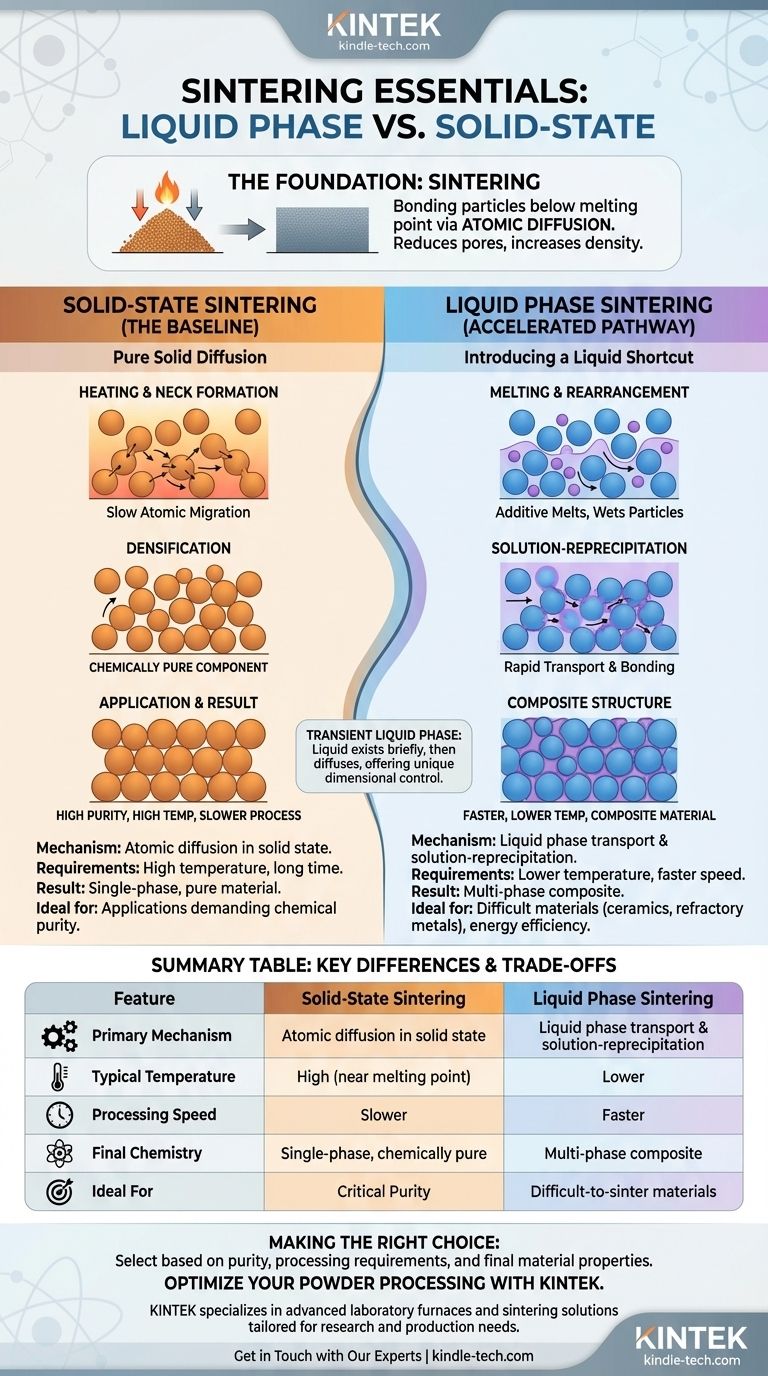
Sintering is a thermal process for compacting a mass of loose powder into a coherent, solid piece. This is achieved by applying heat and often pressure, but at temperatures below the material's melting point.
The primary objective is to dramatically reduce the porous space between the individual particles, squeezing them together until they form a dense, solid object.
The Mechanism: Atomic Diffusion
At the atomic level, sintering works by encouraging atoms to move. Under high heat, atoms migrate from the surface of the powder particles to the points where they touch each other.
This movement of atoms builds "necks" or bridges between particles. As these necks grow, the pores between particles shrink and eventually close, resulting in a densified material.
Solid-State Sintering: The Baseline Process
How It Works: Purely Solid Diffusion
In solid-state sintering, the entire process relies on atoms migrating across solid surfaces. There are no liquid additives involved.
The powder compact is heated to a high temperature, and atoms slowly move to fill the gaps and form strong bonds between adjacent particles, driven entirely by thermal energy and pressure.
Key Requirements: High Temperature & Time
Because atomic diffusion through a solid is a very slow process, solid-state sintering requires significant energy. It demands high temperatures, often approaching the primary material's melting point, and can require long hold times to achieve high density.
The Result: A Chemically Pure Component
A major advantage of solid-state sintering is chemical purity. Since no additives are used, the final sintered part consists only of the original powder material. This is critical for applications where even trace amounts of a second phase would be detrimental.
Liquid Phase Sintering: The Accelerated Pathway
The Core Principle: Introducing a Liquid "Shortcut"
Liquid phase sintering is used for materials that are very difficult to densify via solid-state methods, such as those with extremely high melting points or slow diffusion rates.
A small amount of a second powder (an additive or "sintering aid") with a lower melting point is mixed with the primary powder.
Stage 1: Rearrangement
When the mix reaches a temperature above the additive's melting point but below the primary material's, the additive melts and forms a liquid. This liquid wets the solid particles, and powerful capillary forces pull them together into a much denser packing arrangement.
Stage 2: Solution-Reprecipitation
Next, the solid particles begin to dissolve into the surrounding liquid, particularly at the high-stress contact points between them. This dissolved material is then transported through the liquid and re-precipitates (solidifies) in the low-stress void areas, such as the necks between particles. This process is significantly faster than solid-state diffusion.
A Note on Transient Liquid Phase
In some advanced cases, called transient liquid phase sintering, the liquid exists only for a short time. For example, when sintering iron with a copper additive, the molten copper quickly diffuses into the solid iron particles, strengthening them and then disappearing as a distinct liquid phase.
Understanding the Key Differences & Trade-offs
Temperature and Speed
Liquid phase sintering is significantly faster and more energy-efficient. The presence of a liquid transport medium allows for densification at much lower temperatures and in shorter times compared to the slow, high-temperature demands of solid-state sintering.
Material Compatibility
Liquid phase sintering is often the only practical option for materials like ceramics, cermets (e.g., tungsten carbide-cobalt), and refractory metals. These materials have melting points too high or diffusion rates too low for effective solid-state densification.
Final Microstructure and Chemistry
This is a critical trade-off. Solid-state sintering produces a single-phase, chemically pure part. Liquid phase sintering results in a composite material containing at least two phases: the primary material and the solidified liquid phase, which remains in the microstructure (often at the grain boundaries). This second phase will alter the final mechanical, thermal, and electrical properties.
Process Control & Dimensional Change
The significant material transport in liquid phase sintering can lead to more substantial shrinkage, which must be carefully predicted and controlled. However, advanced methods like transient liquid phase sintering can be engineered to balance natural shrinkage with swelling, resulting in components with near-zero dimensional change during processing.
Making the Right Choice for Your Goal
Selecting the correct sintering path requires understanding the final properties your component needs.
- If your primary focus is chemical purity and a single-phase material: Solid-state sintering is the necessary choice to avoid introducing a secondary binder phase.
- If your primary focus is processing high-melting-point materials or reducing energy costs: Liquid phase sintering is the more efficient and often the only practical approach.
- If your primary focus is precise dimensional control in a multi-material system: Transient liquid phase sintering offers unique engineering advantages to minimize shrinkage or swelling.
- If your primary focus is maximizing speed and density for any material: Advanced heating techniques like Spark Plasma Sintering (SPS) can be used to drive either the solid-state or liquid phase mechanism much more rapidly than conventional furnaces.
Understanding these fundamental mechanisms empowers you to select the right process not just for what you are making, but for the specific performance you need to achieve.
Summary Table:
| Feature | Solid-State Sintering | Liquid Phase Sintering |
|---|---|---|
| Primary Mechanism | Atomic diffusion in solid state | Liquid phase transport & solution-reprecipitation |
| Typical Temperature | High (closer to melting point) | Lower |
| Processing Speed | Slower | Faster |
| Final Chemistry | Single-phase, chemically pure | Multi-phase composite |
| Ideal For | Materials where purity is critical | Difficult-to-sinter materials (e.g., ceramics, refractory metals) |
Optimize Your Powder Processing with KINTEK
Choosing the right sintering method is critical for achieving the desired density, purity, and performance in your components. Whether your project requires the chemical purity of solid-state sintering or the efficiency and capability of liquid phase sintering, having the right equipment is key.
KINTEK specializes in advanced laboratory furnaces and sintering solutions tailored for research and production needs. Our experts can help you select the perfect system to ensure precise temperature control and optimal results for your specific materials.
Contact us today to discuss how our lab equipment can enhance your sintering processes and help you create superior materials.
Visual Guide
Related Products
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
- 600T Vacuum Induction Hot Press Furnace for Heat Treat and Sintering
- Vacuum Dental Porcelain Sintering Furnace
- Graphite Vacuum Furnace IGBT Experimental Graphitization Furnace
People Also Ask
- Does sintering use diffusion? The Atomic Mechanism for Building Stronger Materials
- What is a sputtering machine? A Guide to High-Quality Thin Film Deposition
- What is a magnetron sputtering? A Guide to High-Quality Thin-Film Deposition
- What is magnetron sputtering machine? Precision Thin-Film Deposition for Advanced Materials
- Why is sintering easier in the presence of a liquid phase? Unlock Faster, Lower-Temperature Densification



















