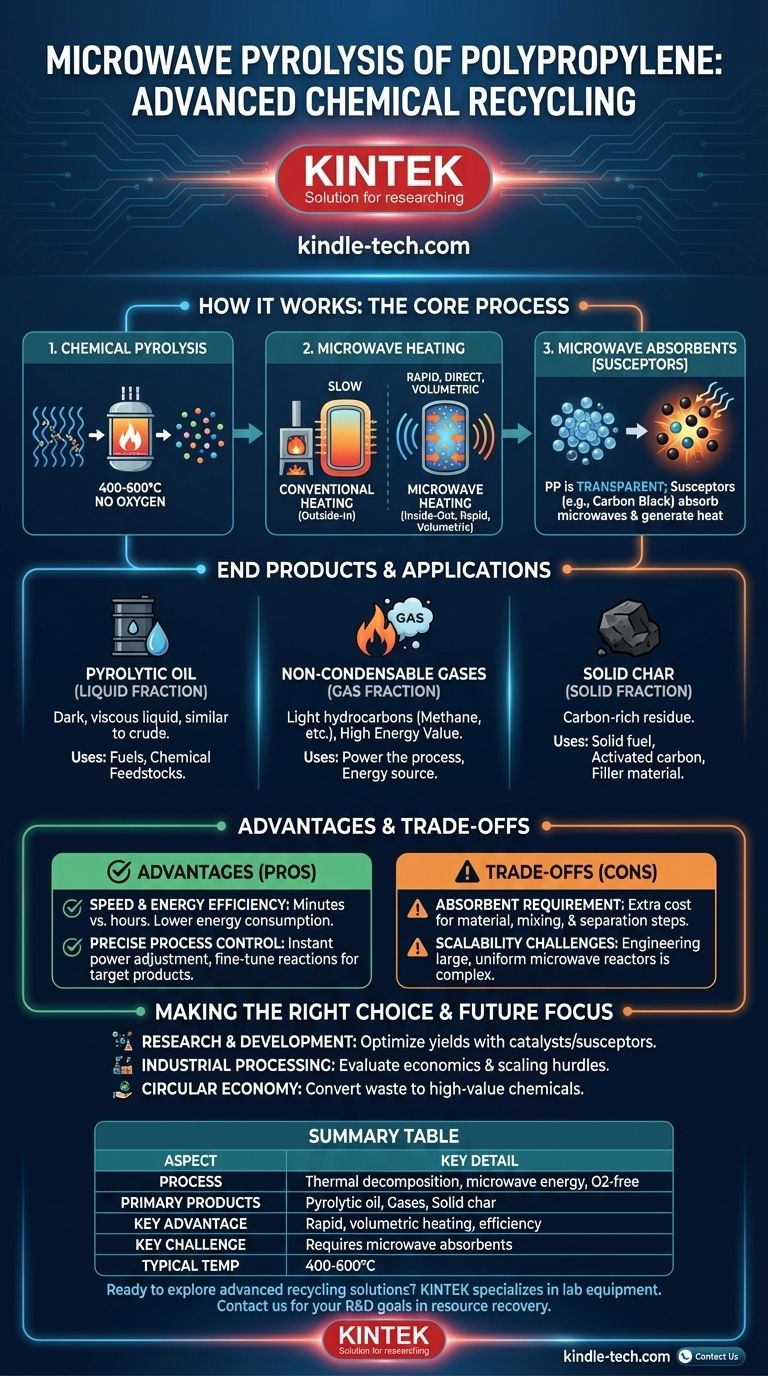
In short, microwave pyrolysis of polypropylene is an advanced chemical recycling technique that uses microwave energy to break down polypropylene plastic waste into valuable products like liquid fuels, gases, and chemical feedstocks. This process occurs in an oxygen-free environment, which prevents the plastic from burning and instead decomposes its long polymer chains into smaller, more useful molecules.
The central advantage of using microwaves is the heating method itself. Unlike conventional furnaces that heat from the outside-in, microwaves provide rapid, direct, and volumetric heating, offering the potential for a more energy-efficient and precisely controlled recycling process.

How Microwave Pyrolysis Works
To fully grasp the technology, it's essential to understand its two core components: the pyrolysis reaction and the unique nature of microwave heating. This combination is what sets the process apart from traditional methods.
The Foundation: Chemical Pyrolysis
Pyrolysis is the thermal decomposition of a material in the complete absence of oxygen. When you heat plastic, it melts. If you keep heating it to very high temperatures (typically 400-600°C) without oxygen, its long, complex polymer chains break apart, or "crack."
This process transforms the solid plastic waste not into ash, but into a mix of smaller, valuable hydrocarbon molecules. It is a fundamental method of chemical recycling.
The Differentiator: Microwave Heating
Conventional pyrolysis uses furnaces that rely on conduction and convection—heating the outside of a reactor and waiting for that heat to slowly penetrate the material inside. Microwave heating is fundamentally different.
Microwaves generate heat from within the material itself. They cause certain molecules to vibrate rapidly, creating friction and, therefore, heat. This results in an incredibly fast and uniform heating process throughout the entire volume of the material.
The Critical Component: Microwave Absorbents
A key challenge is that pure plastics like polypropylene (PP) are largely transparent to microwaves, much like a ceramic dish in your kitchen microwave. They don't heat up efficiently on their own.
To solve this, a microwave-absorbent material, often called a susceptor, is mixed with the plastic. Materials like carbon black, silicon carbide, or certain metal oxides are excellent at absorbing microwave energy and converting it into heat, which is then rapidly transferred to the surrounding plastic, initiating pyrolysis.
What Are the End Products?
The output of polypropylene pyrolysis can be tailored by adjusting process conditions like temperature and heating rate. The primary products fall into three categories.
Pyrolytic Oil (Liquid Fraction)
This is typically the most desired product. It's a dark, viscous liquid composed of a complex mixture of hydrocarbons, similar in composition to crude oil or diesel. This oil can be refined into fuels or used as a feedstock to create new plastics and chemicals.
Non-Condensable Gases (Gas Fraction)
This fraction consists of light hydrocarbon gases like methane, ethane, propane, and hydrogen. While sometimes considered a byproduct, these gases have a high energy value and are often captured and used to power the pyrolysis reactor itself, making the overall process more energy-efficient.
Solid Char (Solid Fraction)
A carbon-rich solid residue, similar to charcoal or carbon black, is also produced. This char can be used as a solid fuel, an adsorbent for filtration (after activation), or as a filler material in asphalt or rubber products.
Understanding the Advantages & Trade-offs
Microwave pyrolysis presents a compelling alternative to traditional methods, but it's important to weigh its benefits against its practical challenges.
Advantage: Speed and Energy Efficiency
Because microwave heating is so rapid and direct, the process can reach target temperatures in minutes rather than hours. This significantly shortens reaction times and can lead to lower overall energy consumption compared to conventional pyrolysis furnaces.
Advantage: Precise Process Control
Microwave power can be adjusted instantly, offering exceptionally fine control over the heating rate and temperature profile. This precision allows operators to better influence the chemical reactions and selectively target the production of more valuable oils or specific chemicals.
Trade-off: The Absorbent Requirement
The need to add a microwave-absorbent material complicates the process. It introduces an extra cost for the material itself and requires an additional step to mix it with the plastic feedstock and potentially separate it from the final solid char.
Trade-off: Scalability Challenges
Designing and scaling large, industrial microwave reactors for continuous operation presents greater engineering challenges than scaling traditional furnaces. Ensuring uniform microwave distribution and managing material flow in a large-scale system is an active area of research and development.
Making the Right Choice for Your Goal
Understanding this technology allows you to see its potential place in the future of recycling and resource management. Its suitability depends entirely on the intended application.
- If your primary focus is research and development: This technology offers a fertile ground for optimizing product yields by experimenting with different catalysts and susceptor materials.
- If your primary focus is industrial waste processing: You must carefully evaluate the economics of the absorbent material and the technical hurdles of scaling the reactor technology for high-throughput operations.
- If your primary focus is a circular economy: View this as a powerful tool for chemical recycling, capable of converting low-value, difficult-to-recycle plastic waste back into high-value chemical building blocks.
Ultimately, microwave pyrolysis of polypropylene represents a significant step toward transforming plastic waste from an environmental burden into a valuable resource.
Summary Table:
| Aspect | Key Detail |
|---|---|
| Process | Thermal decomposition using microwave energy in an oxygen-free environment |
| Primary Products | Pyrolytic oil, non-condensable gases, solid char |
| Key Advantage | Rapid, volumetric heating for speed and energy efficiency |
| Key Challenge | Requires addition of microwave-absorbent materials (e.g., carbon) |
| Typical Temperature | 400-600°C |
Ready to explore advanced recycling solutions for your laboratory or operation? KINTEK specializes in lab equipment and consumables, serving laboratory needs. Our expertise can help you implement efficient processes like pyrolysis. Contact us today to discuss how we can support your research and development goals in chemical recycling and resource recovery.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Microwave Plasma Chemical Vapor Deposition MPCVD Machine System Reactor for Lab and Diamond Growth
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Mini SS High Pressure Autoclave Reactor for Laboratory Use
- Cylindrical Resonator MPCVD Machine System Reactor for Microwave Plasma Chemical Vapor Deposition and Lab Diamond Growth
People Also Ask
- How does the rotational frequency of a graphite drum influence aluminum bronze castings? Master SHS Process Precision
- What is the temperature of carbon reactivation? Optimize Your Process Between 700°C and 900°C
- What are the problems of rotary kiln of cement and their remedies? Achieve Long-Term Reliability and Efficiency
- What is the pyrolysis reaction of biomass? A Guide to Converting Waste into Valuable Biochar, Bio-oil, and Syngas
- What is the temperature range of a rotary kiln? A Guide to Custom Thermal Profiles
- How is cement prepared by rotary kiln method? A Step-by-Step Guide to Clinker Production
- What is the temperature and time of pyrolysis? Control Your Product Output with Precision
- What is a rotary kiln? A Guide to High-Temperature Material Processing



















