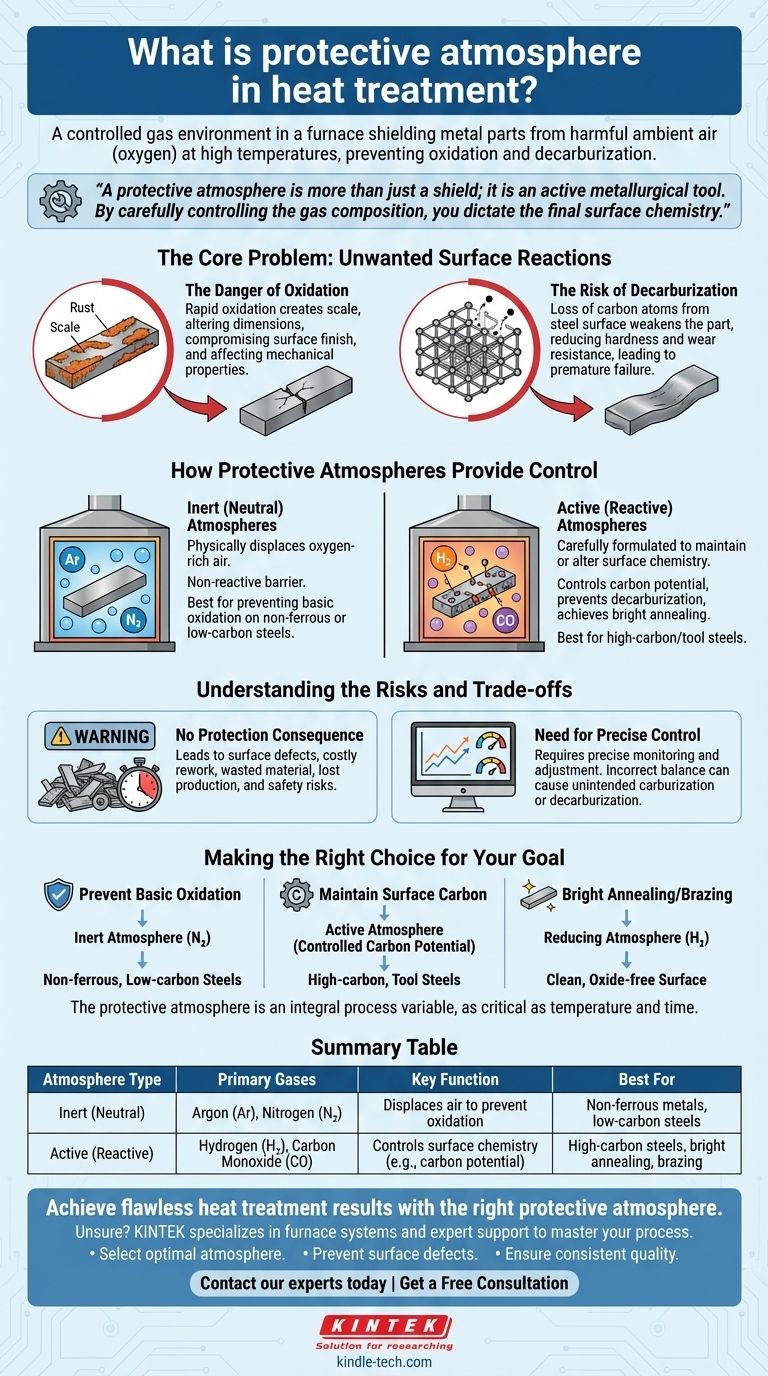
In heat treatment, a protective atmosphere is a specifically engineered and controlled gas environment that surrounds a metal part inside a furnace. Its fundamental purpose is to shield the component from the harmful effects of ambient air—primarily oxygen—at high temperatures, thereby preventing unwanted surface reactions like oxidation (scaling) and decarburization.
A protective atmosphere is more than just a shield; it is an active metallurgical tool. By carefully selecting and controlling the gas composition, you can dictate the final surface chemistry of a part, ensuring it meets precise engineering requirements for strength, hardness, and durability.

The Core Problem: Unwanted Surface Reactions
Heat treatment relies on high temperatures to alter a metal's internal structure. However, this same heat dramatically accelerates chemical reactions between the metal's surface and any gases present in the furnace.
The Danger of Oxidation
When heated in the presence of oxygen (from air), most metals will rapidly oxidize. This creates a layer of scale or rust on the surface.
This oxidation is not just a cosmetic issue. It can alter the dimensions of a precision part, compromise its surface finish, and in severe cases, negatively affect its mechanical properties.
The Risk of Decarburization
For carbon steels, another significant risk is decarburization. This is the loss of carbon atoms from the surface of the steel.
Since carbon is the primary element that gives steel its hardness and strength, losing it from the surface makes the part weaker and less wear-resistant than intended. This is a critical failure that can lead to premature component failure.
How Protective Atmospheres Provide Control
Protective atmospheres are broadly categorized by how they interact with the workpiece. They can be either neutral, simply displacing air, or active, creating a specific chemical reaction at the surface.
Inert (Neutral) Atmospheres
The simplest form of protection involves using an inert gas to physically displace the oxygen-rich air.
Gases like Argon (Ar) and Nitrogen (N2) are used for this purpose. They are non-reactive with the metal and serve as a simple, effective barrier against oxidation for many common processes.
Active (Reactive) Atmospheres
More advanced applications require active atmospheres, which are carefully formulated gas mixtures designed to maintain or even alter the surface chemistry.
These atmospheres, often containing gases like hydrogen (H2), carbon monoxide (CO), and precisely controlled levels of others, can achieve specific goals. They can be adjusted to create a "reducing" environment that strips away light oxides or to match the carbon potential of the steel, actively preventing decarburization.
Understanding the Risks and Trade-offs
Failing to implement a proper protective atmosphere is not a viable cost-saving measure; it is a direct risk to product quality and operational efficiency.
The Consequence of No Protection
Processing parts without a controlled atmosphere leads directly to surface defects. This results in parts that fail quality inspection, requiring costly rework or being scrapped entirely.
The consequences ripple outward, causing wasted material, lost production time, and—if a faulty part enters the supply chain—a significant safety risk to the end-user.
The Need for Precise Control
Using a protective atmosphere is not a "set and forget" process. An incorrectly balanced gas mixture can be just as damaging as using no protection at all.
For example, an active atmosphere with the wrong carbon potential can cause unintended carburization (adding too much carbon) or decarburization. This requires precise control systems to monitor and adjust the gas composition throughout the heat treatment cycle.
Making the Right Choice for Your Goal
The choice of atmosphere is dictated entirely by the material being treated and the desired outcome of the process.
- If your primary focus is preventing basic oxidation on non-ferrous or low-carbon steel parts: A simple inert atmosphere of nitrogen is often the most effective and economical solution.
- If your primary focus is maintaining the precise surface carbon of high-carbon or tool steels: An active atmosphere with a controlled carbon potential is essential to prevent decarburization.
- If your primary focus is bright annealing or brazing that requires an exceptionally clean, oxide-free surface: A reducing atmosphere containing hydrogen is necessary to chemically remove surface oxides.
Ultimately, the protective atmosphere must be considered an integral process variable, just as critical as temperature and time.
Summary Table:
| Atmosphere Type | Primary Gases | Key Function | Best For |
|---|---|---|---|
| Inert (Neutral) | Argon (Ar), Nitrogen (N₂) | Displaces air to prevent oxidation | Non-ferrous metals, low-carbon steels |
| Active (Reactive) | Hydrogen (H₂), Carbon Monoxide (CO) | Controls surface chemistry (e.g., carbon potential) | High-carbon steels, bright annealing, brazing |
Achieve flawless heat treatment results with the right protective atmosphere.
Unsure which atmosphere is best for your material and process goals? The wrong choice can lead to costly scrap, rework, and part failure. KINTEK specializes in lab equipment and consumables, providing the precise furnace systems and expert support you need to master your heat treatment processes.
We can help you:
- Select the optimal atmosphere for your specific metal and application.
- Prevent surface defects like scaling and decarburization.
- Ensure consistent, high-quality results batch after batch.
Contact our experts today to discuss your requirements and ensure your parts meet the highest standards of strength and durability.
Visual Guide
Related Products
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- Vertical Laboratory Tube Furnace
- Vacuum Heat Treat Sintering Brazing Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Graphite Vacuum Furnace High Thermal Conductivity Film Graphitization Furnace
People Also Ask
- What is the preferred firing environment for high-volume, low-carbon stainless steel parts? Optimize MIM & Pressing
- What role does an atmosphere furnace utilizing hydrogen gas play in Cu-Cr-Nb alloy powder pretreatment? (Key Insights)
- What is a controlled atmosphere temperature treatment system? A Guide to Precision Heat Treatment
- What is the role of a precision heat treatment furnace in the annealing of nanostructured eutectic steel?
- What is the function of a high-temperature atmosphere furnace in biochar preparation? Engineer Effective Adsorbents
- What are the needs of annealing process? A Guide to Relieving Stress and Restoring Ductility
- Why is a high-purity Argon environment required during aluminum powder oxidation preheating? Ensure Data Accuracy
- How to create an inert atmosphere in a furnace? Master the Vacuum-Purge Method for Oxidation-Free Results



















