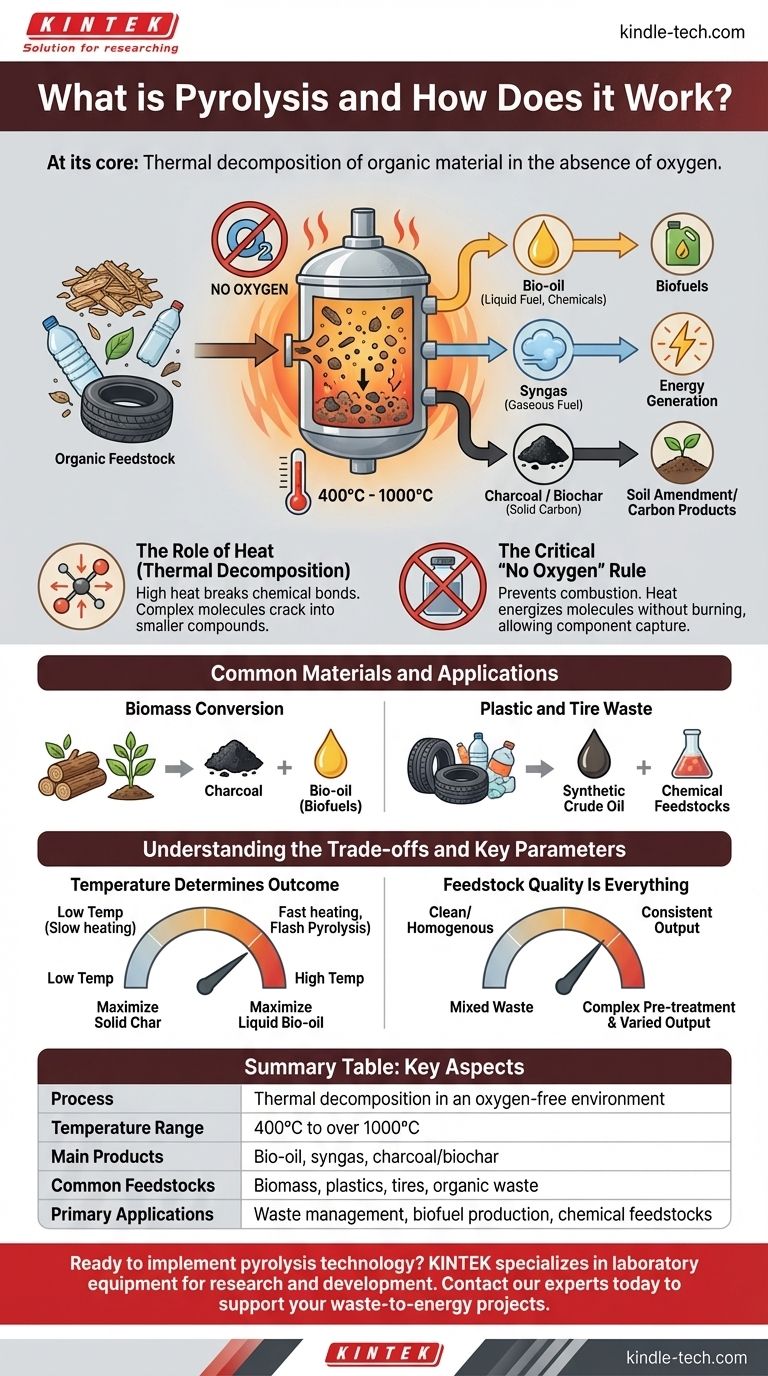
At its core, pyrolysis is the thermal decomposition of organic material in the absence of oxygen. Instead of burning a substance like plastic or wood, which requires oxygen, pyrolysis uses high heat in a controlled, oxygen-free environment to break it down into smaller, often more valuable molecules.
Pyrolysis should not be confused with burning. It is a precise thermochemical process that deconstructs complex organic materials into their fundamental components, transforming potential waste into useful resources like liquid fuels and charcoal.

The Core Mechanism: How Pyrolysis Works
To understand pyrolysis, you must grasp two key principles: the role of intense heat and the absolute necessity of an oxygen-free environment. These two factors are what separate it from simple combustion.
The Role of Heat (Thermal Decomposition)
Pyrolysis works because the chemical bonds holding large organic molecules together have limited thermal stability. When exposed to high temperatures, typically ranging from 400°C to over 1000°C depending on the goal, these bonds vibrate and break apart.
This process is known as thermal decomposition or cracking. The large, complex molecules in the original material are "cracked" into a mix of smaller, less complex molecules in solid, liquid, and gaseous states.
The Critical "No Oxygen" Rule
The absence of oxygen is the defining characteristic of pyrolysis. If oxygen were present, the material would simply ignite and burn (combust), releasing energy as heat and light while producing ash and emissions like carbon dioxide.
By removing oxygen, we prevent combustion. The heat energy doesn't burn the material; it energizes the molecules until they break apart, allowing us to capture the resulting components.
From Feedstock to New Products
The process begins with an organic feedstock—the raw material being processed. This material is fed into a sealed reactor, which is then heated by a precisely controlled system.
As the material decomposes, it separates into different substances. The exact output depends heavily on the feedstock and process conditions, but the goal is to create useful products like an intermediate liquid fuel or a solid carbon residue like charcoal.
Common Materials and Applications
Pyrolysis is a versatile technology applicable to a wide range of carbon-based materials, making it a key process in waste valorization and biofuel production.
Biomass Conversion
Pyrolysis is used to convert biomass, such as wood waste or agricultural scraps, into valuable products. The most classic example is the production of charcoal from wood by heating it in a low-oxygen kiln.
More advanced systems use pyrolysis to convert biomass into a liquid product known as bio-oil, which can be refined into hydrocarbon biofuels and other chemical additives.
Plastic and Tire Waste
Pyrolysis plants are increasingly used to address plastic and rubber waste. This is particularly valuable for mixed or contaminated plastics that are difficult to recycle mechanically.
The process breaks down the long polymer chains in plastics and tires, converting them back into smaller hydrocarbon molecules. This can produce a synthetic crude oil that serves as a fuel or a feedstock for the chemical industry.
Understanding the Trade-offs and Key Parameters
While powerful, pyrolysis is not a simple solution. It is a sophisticated industrial process where precise control over key variables is essential for a successful outcome.
Temperature Determines the Outcome
The temperature inside the pyrolysis reactor is the most critical control parameter. Different temperatures and heating rates favor the production of different outputs.
For example, a slow heating process might maximize the production of solid char, while a very fast process ("flash pyrolysis") is often used to maximize the yield of liquid bio-oil. Precision temperature control, often using PID monitoring systems, is non-negotiable.
Feedstock Quality Is Everything
The composition of the input material directly impacts the quality and quantity of the output products. The moisture content, physical size, and chemical makeup of the feedstock must be managed.
Processing a clean, homogenous material like wood chips is far different from processing mixed municipal solid waste. The latter requires more complex pre-treatment and results in a more varied output that may need further refinement.
Making the Right Choice for Your Goal
Understanding pyrolysis allows you to see it not as a single process, but as a versatile platform for transforming materials. Your specific objective will determine how you apply it.
- If your primary focus is waste management: Pyrolysis is a powerful technology for converting non-recyclable materials like mixed plastics and tires into a more stable, usable form.
- If your primary focus is energy production: The process is a key pathway for creating intermediate liquid fuels from biomass and other organic waste streams.
- If your primary focus is material production: Pyrolysis is the fundamental chemical process behind creating specific carbon-based products like charcoal.
Ultimately, pyrolysis is a technology that embodies the principles of a circular economy by chemically deconstructing low-value materials to create high-value resources.
Summary Table:
| Key Aspect | Description |
|---|---|
| Process | Thermal decomposition in an oxygen-free environment |
| Temperature Range | 400°C to over 1000°C |
| Main Products | Bio-oil, syngas, charcoal/biochar |
| Common Feedstocks | Biomass, plastics, tires, organic waste |
| Primary Applications | Waste management, biofuel production, chemical feedstocks |
Ready to implement pyrolysis technology in your operations? KINTEK specializes in laboratory equipment and consumables for pyrolysis research and development. Whether you're working with biomass conversion, plastic waste recycling, or biofuel production, our precision heating systems and reactors can help you optimize your pyrolysis processes. Contact our experts today to discuss how we can support your waste-to-energy projects with reliable, high-performance laboratory solutions.
Visual Guide
Related Products
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Vertical Laboratory Tube Furnace
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
People Also Ask
- What temperature is needed for pyrolysis waste? A Guide to Optimizing Your Waste-to-Value Process
- What is the range of pyrolysis? Master Temperature Control for Optimal Bio-Product Yields
- What are the process advantages of using a rotary tube furnace for WS2 powder? Achieve Superior Material Crystallinity
- How is a high-temperature calcination furnace utilized in BZY20 Sol-gel? Achieve Pure Cubic Perovskite Phases
- Why are high temperatures required when sintering stainless steels? Unlock Pure, High-Density Results



















