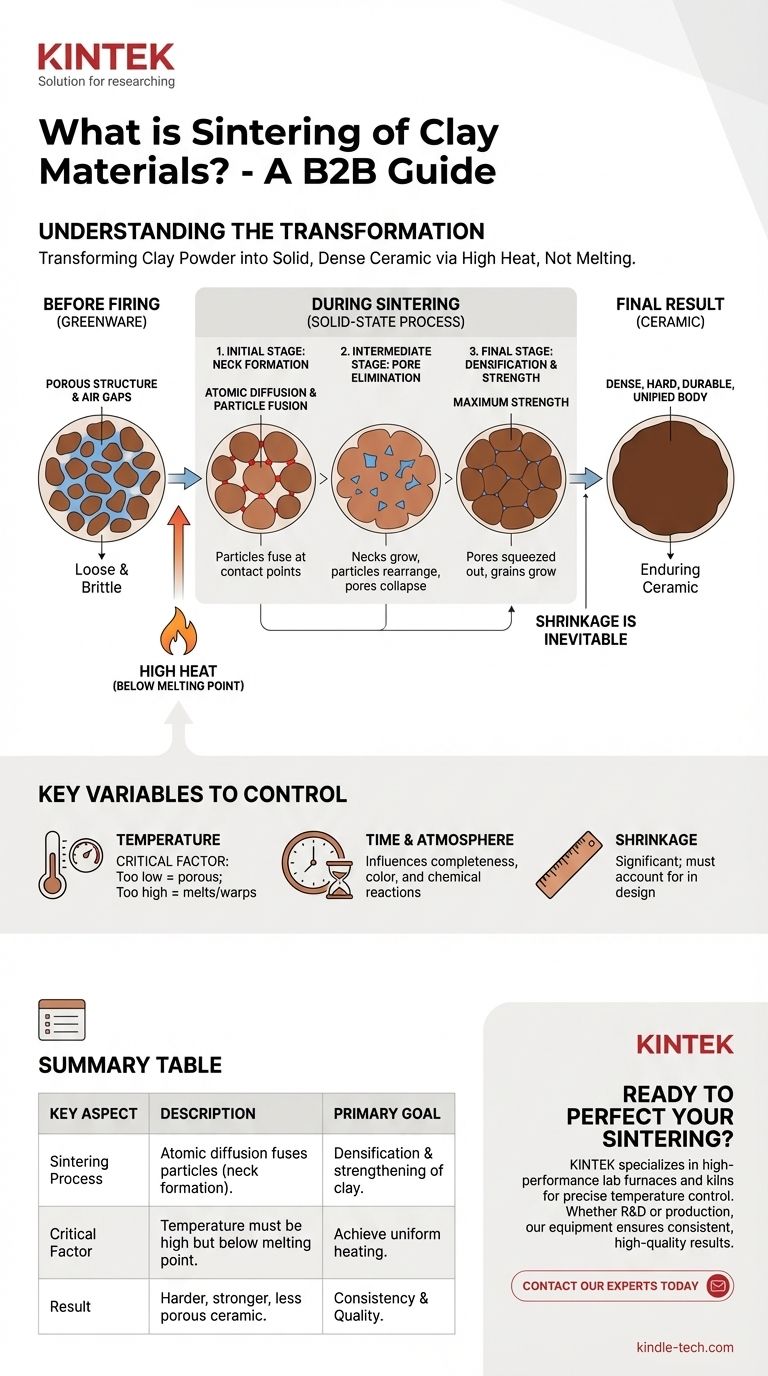
In essence, sintering is the process of transforming a powder-like material, such as clay, into a solid, dense object using high heat. This is achieved by heating the material to a temperature below its melting point, which causes the individual particles to fuse together at their contact points. This atomic-level bonding eliminates the empty spaces between particles, resulting in a harder, stronger, and more durable final ceramic piece.
The crucial concept to understand is that sintering is not melting. It is a solid-state process where heat energizes the atoms in clay particles, causing them to diffuse and bond across their boundaries, systematically removing porosity and creating a unified, strong ceramic body.

How Sintering Fundamentally Transforms Clay
To truly grasp sintering, you must visualize the change happening at a microscopic level. It's a journey from a loose collection of particles to a single, integrated mass.
The Starting Point: A Porous Structure
Before firing, an object made of dried clay (known as greenware) is simply a compacted mass of individual clay particles. It is brittle and filled with countless tiny air gaps, or pores, making it highly porous and weak.
The Role of Heat (Not Melting)
When placed in a kiln, the clay is heated to a high temperature, but one that is carefully controlled to remain below the clay's liquefaction point. This heat doesn't turn the clay into a liquid; instead, it provides the energy necessary to activate atomic movement within the solid particles.
Atomic Diffusion and Particle Fusion
This added energy allows atoms on the surface of the clay particles to become mobile. They begin to migrate across the boundaries where particles touch, a process called atomic diffusion. This migration effectively builds bridges, or "necks," between adjacent particles, fusing them together into a single, connected network.
The Final Result: Densification and Strength
As these connections grow, the particles pull closer together, systematically shrinking and closing the pores between them. This process, known as densification, is the primary goal of sintering. By eliminating the empty space, the material becomes significantly denser, harder, and stronger, transforming the fragile clay into durable ceramic.
The Key Stages of Ceramic Sintering
The transformation from powder to solid ceramic generally occurs in three overlapping stages.
Initial Stage: Neck Formation
At the very beginning of the process, the points where individual particles touch begin to fuse. These initial bonds, or necks, start to link the loose powder into a cohesive, albeit still very porous, structure.
Intermediate Stage: Pore Elimination
As the temperature holds or rises, the necks grow larger and the particles rearrange to pack more tightly. The network of interconnected pores collapses into smaller, isolated pockets of trapped gas. During this stage, the ceramic body undergoes most of its shrinkage and densification.
Final Stage: Grain Growth
In the last stage, the remaining isolated pores are squeezed out, and the individual crystal grains within the ceramic may begin to grow larger. This completes the densification process, resulting in a strong ceramic body with minimal porosity and maximum strength.
Understanding the Key Variables
Sintering is a precise process. Controlling the variables is essential to achieving the desired outcome, as small changes can have significant effects on the final product.
Temperature is Critical
The temperature profile is the most important factor. If the temperature is too low, sintering will be incomplete, leaving the piece weak and porous. If the temperature is too high, the material can begin to melt, leading to warping, bloating, or the complete collapse of the object in the kiln.
Time and Atmosphere Matter
The amount of time the ceramic spends at the peak temperature directly influences how completely it sinters. Furthermore, the chemical composition of the kiln's atmosphere (e.g., rich in oxygen or deprived of it) can affect the chemical reactions during sintering, impacting the color and other properties of the final piece.
Shrinkage is Inevitable
Because sintering works by eliminating the empty space between particles, the entire object will shrink. Ceramicists must account for this shrinkage, which can be significant, during the initial design and forming of the piece.
Applying This to Your Ceramic Goal
Understanding the principles of sintering allows you to control the outcome of your work with intention.
- If your primary focus is functional, watertight pottery (e.g., a mug or bowl): Your goal is full sintering to achieve vitrification, which closes all pores and makes the ceramic impervious to water.
- If your primary focus is creating porous ceramics (e.g., a terracotta plant pot or a water filter): You will use lower firing temperatures to deliberately achieve only partial sintering, preserving a network of open pores.
- If your primary focus is specific artistic effects: Manipulating temperature, time, and kiln atmosphere allows you to precisely control the final texture, strength, and color of your finished ceramic piece.
Ultimately, mastering sintering is mastering the fundamental transformation from raw earth into enduring ceramic art and technology.
Summary Table:
| Key Aspect | Description |
|---|---|
| Primary Goal | Densification & strengthening of clay by eliminating pores. |
| Key Process | Atomic diffusion fuses particles at contact points (neck formation). |
| Critical Factor | Temperature must be high but below the clay's melting point. |
| Result | A harder, stronger, and less porous ceramic object. |
Ready to perfect your ceramic sintering process? KINTEK specializes in high-performance lab furnaces and kilns that deliver the precise temperature control and uniform heating essential for consistent, high-quality results. Whether you're in R&D or production, our equipment is designed to meet your exact sintering needs. Contact our experts today to find the ideal solution for your laboratory!
Visual Guide
Related Products
- 1800℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- What is the tolerance of a muffle furnace? A Guide to Temperature Accuracy & Uniformity
- What is the heat transfer of a muffle furnace? Understanding Indirect Heating for Purity
- What is the highest temperature of a furnace? Unlocking the Limits of Extreme Heat
- Why do we use a muffle furnace? For Pure, Precise, and Contaminant-Free High-Temperature Processing
- What is the primary use of furnace in the chemical industry? Master Thermal Treatment for Material Transformation



















