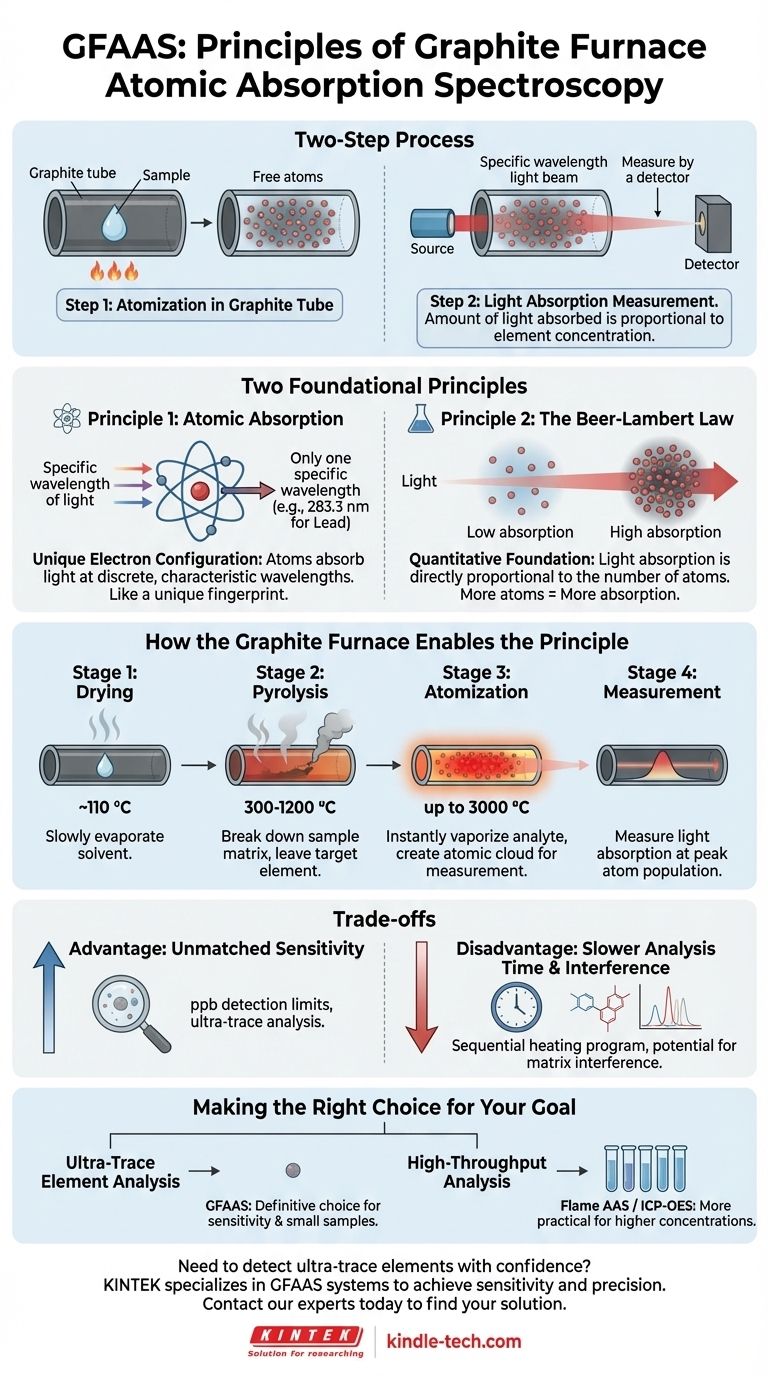
At its core, the principle of Graphite Furnace Atomic Absorption Spectroscopy (GFAAS) is a two-step process. First, a sample is heated in a graphite tube to create a contained cloud of free, neutral atoms. Second, a beam of light specific to the element being measured is passed through this cloud, and the amount of light absorbed is directly proportional to the concentration of the element in the sample.
The key principle is not just that atoms absorb light, but that the graphite furnace provides a highly efficient and controlled environment to convert the entire sample into an atomic vapor, trapping it briefly in the light path for maximum sensitivity.

The Two Foundational Principles
GFAAS operates on two well-established scientific laws that work in tandem. Understanding both is essential to grasping how the technique achieves its remarkable precision.
Principle 1: Atomic Absorption
Every element has a unique electron configuration. Because of this, atoms of a specific element will only absorb light at very discrete, characteristic wavelengths.
This phenomenon acts like a unique fingerprint. For example, lead atoms will only absorb light at 283.3 nm, while copper atoms absorb at 324.8 nm. GFAAS exploits this by using a light source that emits the precise wavelength for the element of interest.
Principle 2: The Beer-Lambert Law
This law provides the quantitative foundation for the measurement. It states that the amount of light absorbed by the atomic cloud is directly proportional to the number of atoms in the light path.
In simple terms: more atoms mean more light absorption. By measuring the decrease in light intensity as it passes through the sample, we can accurately determine the concentration of the target element.
How the Graphite Furnace Enables the Principle
The "graphite furnace" is the atomizer—the component responsible for creating the cloud of free atoms from the initial liquid or solid sample. Its design and programmed heating are what make the technique so powerful.
The Graphite Tube as a Micro-Furnace
The heart of the instrument is a small, hollow graphite tube. The sample (typically a few microliters) is placed inside this tube.
The tube is positioned so a beam of light can pass directly through its center. It is also connected to electrodes that can pass a high current through it, heating it resistively to temperatures up to 3000 °C in seconds.
The Controlled Heating Program
Unlike a simple flame, the furnace follows a precise, multi-step temperature program to prepare the sample for measurement.
- Drying: The tube is first heated gently (e.g., ~110 °C) to slowly evaporate the solvent without sputtering the sample.
- Pyrolysis (Ashing): The temperature is increased significantly (e.g., 300-1200 °C) to break down and remove the sample matrix (like organic matter or complex salts) while leaving the target element behind.
- Atomization: For a few seconds, the temperature is rapidly raised to its maximum. This intense heat instantly vaporizes the analyte, creating a dense, localized cloud of free, ground-state atoms directly in the light path.
- Measurement: The instrument measures the light absorption only during this brief atomization step when the atom population is at its peak.
Understanding the Trade-offs
The graphite furnace method offers incredible benefits, but it's essential to understand its operational context and limitations.
Advantage: Unmatched Sensitivity
The primary advantage of GFAAS is its sensitivity. By containing the entire atomized sample in a small area for a couple of seconds, it achieves detection limits thousands of times lower than other methods like Flame AAS, often in the parts-per-billion (ppb) range.
Disadvantage: Slower Analysis Time
The sequential nature of the heating program (dry, pyrolyze, atomize, cool down) means each sample run takes several minutes. This makes GFAAS significantly slower than techniques that can analyze samples continuously.
Disadvantage: Potential for Interference
The complex environment inside the furnace can lead to chemical and spectral interferences from the sample matrix. These must be carefully managed through method development and the use of background correction technologies to ensure accurate results.
Making the Right Choice for Your Goal
Selecting the correct analytical technique depends entirely on your objective.
- If your primary focus is ultra-trace element analysis: GFAAS is the definitive choice due to its exceptional sensitivity and the very small sample volume it requires.
- If your primary focus is high-throughput analysis of many samples: A faster technique like Flame AAS or ICP-OES is more practical, provided your element concentrations are high enough for their detection limits.
By understanding the principle of controlled, total atomization, you can leverage the power of GFAAS when ultimate analytical sensitivity is the critical requirement.
Summary Table:
| Key Aspect | Description |
|---|---|
| Core Principle | Two-step process: atomize sample in a graphite furnace, then measure light absorption by free atoms. |
| Quantitative Law | Beer-Lambert Law: light absorption is proportional to element concentration. |
| Primary Advantage | Unmatched sensitivity for ultra-trace analysis (parts-per-billion range). |
| Key Consideration | Slower analysis time per sample compared to flame AAS or ICP-OES. |
Need to detect ultra-trace elements with confidence? KINTEK specializes in laboratory equipment, including atomic absorption spectrometers. Our experts can help you select the right GFAAS system to achieve the sensitivity and precision your lab demands. Contact our team today to discuss your specific analytical challenges and find the perfect solution.
Visual Guide
Related Products
- Ultra-High Temperature Graphite Vacuum Graphitization Furnace
- Graphite Vacuum Furnace High Thermal Conductivity Film Graphitization Furnace
- Graphite Vacuum Continuous Graphitization Furnace
- Vertical High Temperature Graphite Vacuum Graphitization Furnace
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
People Also Ask
- What is the use of graphite furnace? Achieve Extreme-Temperature Processing for Advanced Materials
- What is the function of the graphite furnace? Achieve Extreme Heat for Analysis & Materials Processing
- What is the principle of graphite furnace? Achieve Extreme Temperatures with Direct Resistive Heating
- What are the advantages of graphite? Unlock Superior Performance in High-Temperature Processes
- What is the temperature of graphite furnace atomic absorption spectrometry? Mastering the Multi-Stage Heating Program
- What is the temperature range of a graphite furnace? Unlock up to 3000°C for advanced materials processing.
- Why is a graphite furnace rather than a flame often used for atomization? Superior Sensitivity for Trace Analysis
- Why is graphite the best conductor of heat? Understanding Its Directional Thermal Superiority



















