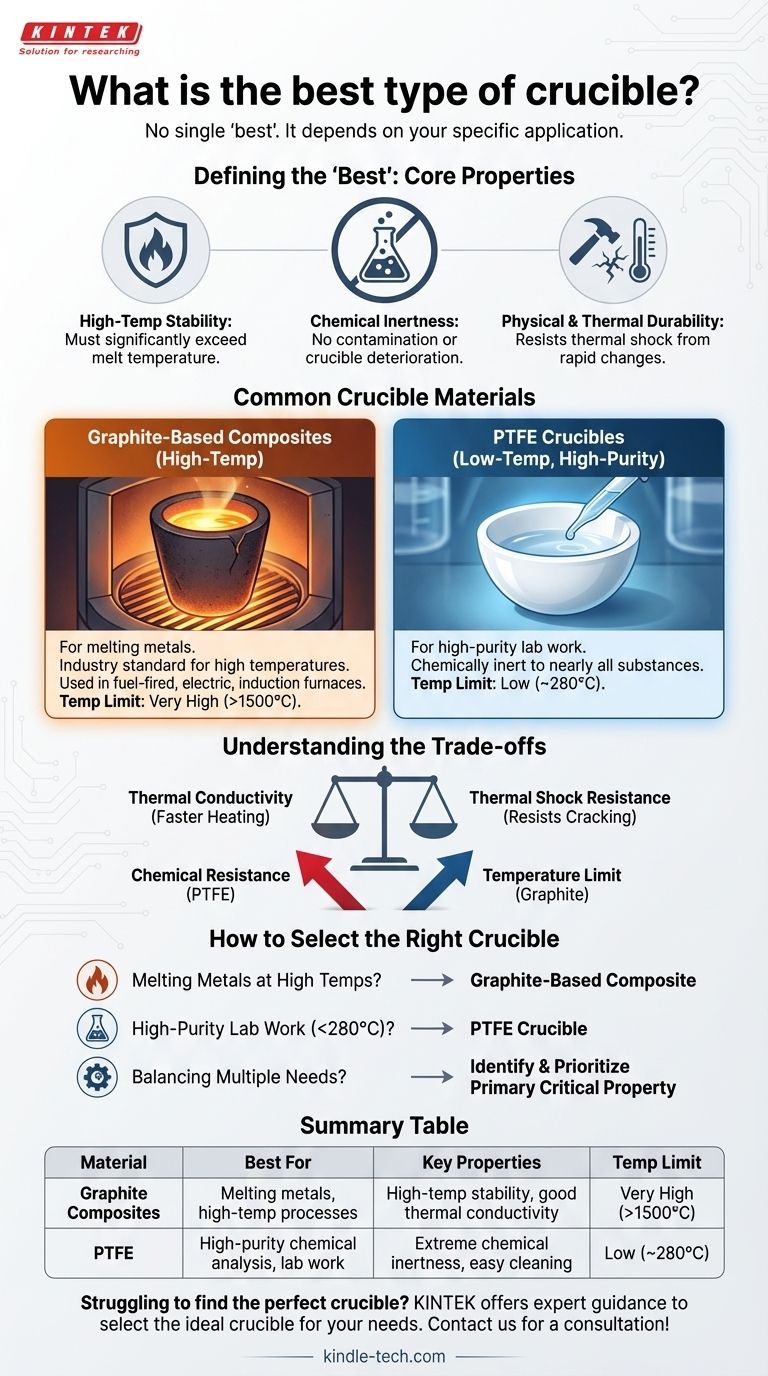
Ultimately, there is no single "best" type of crucible. The ideal crucible is entirely dependent on your specific application. The material you are heating, the maximum temperature you need to reach, and the type of furnace you are using are the critical factors that dictate the right choice for the job.
The process of selecting a crucible is not about finding a universally superior material, but about understanding the necessary trade-offs. The "best" crucible is the one whose properties are most closely aligned with the unique demands of your work.

Defining the "Best" Crucible: Core Properties
To select the right crucible, you must first understand the fundamental properties that define its performance. The ideal choice will provide the optimal balance of these characteristics for your specific process.
High-Temperature Stability
A crucible’s most basic requirement is that its melting point is significantly higher than the material it is designed to hold. It must maintain its structural integrity without degrading, softening, or failing at the highest operating temperatures of your process.
Chemical Inertness
The crucible must be chemically compatible with the melt. Any reaction between the crucible and the material being heated can lead to two problems: contamination of your melt and deterioration of the crucible itself, reducing its lifespan.
Physical and Thermal Durability
A crucible must be robust enough to handle the physical stresses of the process. This includes having outstanding resistance to thermal shock—the cracking that can occur from rapid changes in temperature during heating and cooling cycles.
Common Crucible Materials and Their Roles
Different materials are engineered to excel in different environments. The two main categories highlighted by modern applications are graphite-based composites for high-temperature work and polymers like PTFE for high-purity, low-temperature tasks.
Graphite-Based Composites
For melting metals and other high-temperature applications, graphite-based composites are the industry standard. These are not pure graphite but highly engineered materials whose performance depends on their specific composition and the structural alignment of the graphite within them. They are used in fuel-fired, electric resistance, and induction furnaces.
PTFE Crucibles
For laboratory work requiring extreme chemical purity at lower temperatures, PTFE (polytetrafluoroethylene) crucibles are an excellent choice. They are chemically inert to nearly all substances and have a maximum use temperature of around 280°C, making them unsuitable for melting metals but perfect for certain chemical analyses.
Understanding the Trade-offs
Crucible performance is a game of compromise. Improving one property often comes at the expense of another, which is why identifying your top priority is essential.
Thermal Conductivity vs. Thermal Shock Resistance
A crucible with very high thermal conductivity will heat your melt efficiently, saving time and energy. However, this same property can sometimes make it more susceptible to thermal shock. A material that resists thermal shock better may not transfer heat as quickly.
Chemical Resistance vs. Temperature Limit
This is the classic trade-off illustrated by comparing PTFE and graphite. PTFE offers near-perfect chemical resistance but fails at temperatures well below what is needed for metallurgy. Graphite composites, on the other hand, withstand extreme heat but their chemical compatibility must be carefully matched to the specific metal or alloy being melted.
How to Select the Right Crucible for Your Application
Instead of searching for a single "best" crucible, use your application's needs to guide your decision.
- If your primary focus is melting metals at high temperatures: A graphite-based composite crucible designed for your specific furnace type and alloy is the correct choice.
- If your primary focus is high-purity lab work below 280°C: A PTFE crucible is likely your best option due to its superior chemical inertness and ease of cleaning.
- If your primary focus is balancing multiple performance needs: You must first identify the single most critical property for your process—be it thermal shock resistance, heating speed, or chemical stability—and discuss this priority with your supplier.
Making an informed choice begins with understanding that the best crucible is the one that is engineered for your specific goal.
Summary Table:
| Crucible Material | Best For | Key Properties | Temperature Limit |
|---|---|---|---|
| Graphite Composites | Melting metals, high-temperature processes | High-temperature stability, good thermal conductivity | Very High (>1500°C) |
| PTFE | High-purity chemical analysis, lab work | Extreme chemical inertness, easy cleaning | Low (~280°C) |
Struggling to find the perfect crucible for your specific process? KINTEK specializes in lab equipment and consumables, offering expert guidance to help you select the ideal crucible that balances high-temperature stability, chemical resistance, and thermal shock durability for your laboratory needs. Let our specialists ensure your process runs efficiently and safely. Contact us today for a personalized consultation!
Visual Guide
Related Products
- Custom Machined and Molded PTFE Teflon Parts Manufacturer with PTFE Crucible and Lid
- Arc-Shaped Alumina Ceramic Crucible High Temperature Resistant for Engineering Advanced Fine Ceramics
- High Purity Pure Graphite Crucible for Evaporation
- Alumina Al2O3 Ceramic Crucible Semicircle Boat with Lid for Engineering Advanced Fine Ceramics
- Engineering Advanced Fine Ceramics Alumina Al2O3 Crucible With Lid Cylindrical Laboratory Crucible
People Also Ask
- What is the temperature range of an aluminum crucible? Ensure Accurate Thermal Analysis in Your Lab
- When were crucibles used? From Ancient Metallurgy to Modern Labs
- What is the primary function of high-alumina crucibles in pretreatment? Ensure Safe & Pure Phosphate Glass Synthesis
- Why are alumina crucibles used for Al-LLZ sintering? The Secret to Stable Cubic Phase Lithium Garnet
- How much heat can a ceramic crucible take? Find the Right Crucible for Your High-Temp Process
- What materials can be used as a crucible? Select the Right Material for Your High-Temperature Application
- Can you melt copper in a graphite crucible? Yes, Here's the Proven Method
- Why are platinum crucibles required for fusion experiments? Essential Tools for Rare Earth Element Analysis



















