The growth of carbon nanotubes (CNTs) is fundamentally a catalytic process, most commonly driven by nanoparticles of specific transition metals. The primary catalysts used in virtually all commercial production methods are iron (Fe), cobalt (Co), and nickel (Ni), often used individually or as alloys. These metallic particles are the critical component that enables the formation of the nanotube structure from a carbon source.
While process parameters like temperature and carbon source are important, the catalyst is the true heart of CNT synthesis. It is not merely an initiator; the physical size and chemical state of the catalyst nanoparticle directly template the diameter and structural quality of the nanotube itself.
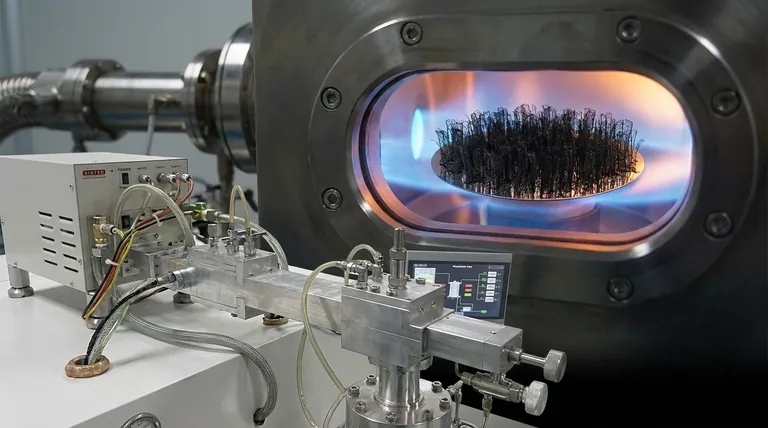
How Metallic Catalysts Drive CNT Formation
Understanding the catalyst's role is understanding the core mechanism of how a CNT is born. The process is not a simple chemical reaction but a complex physical assembly at the nanoscale.
The Role of Catalyst Nanoparticles
The catalyst is not used as a block of bulk metal. Instead, it's prepared as a layer of discrete, nanometer-sized particles, typically deposited on a support substrate like silicon oxide or alumina. These tiny metallic islands are the individual reactors where each CNT will grow.
Step 1: Decomposing the Carbon Source
During synthesis, a carbon-containing gas (a hydrocarbon like methane, ethylene, or acetylene) is introduced at high temperatures (typically 600-1200°C). When this gas flows over the heated catalyst nanoparticles, the metal's surface breaks the hydrocarbon molecules apart, freeing carbon atoms.
Step 2: Carbon Dissolution and Precipitation
The liberated carbon atoms dissolve into and diffuse through the metal nanoparticle, creating a supersaturated solution of carbon in metal. To achieve a more stable state, the carbon precipitates out from the particle. Under the right conditions, this carbon crystallizes not as graphite or diamond, but as a cylindrical tube—the carbon nanotube.
Key Factors Influencing Catalyst Performance
The choice of catalyst and the conditions under which it operates are the most critical parameters controlling the final product. While the references mention temperature and concentration, these factors are only meaningful in the context of how they affect the catalyst.
Catalyst Material (Fe, Co, Ni)
Iron, cobalt, and nickel are uniquely effective because they have a specific set of properties. They have a moderate carbon solubility and a high diffusion rate for carbon at elevated temperatures, which is essential for the dissolution-precipitation mechanism.
Catalyst Size and Diameter Control
This is a crucial concept: the diameter of the catalyst nanoparticle directly dictates the diameter of the CNT that grows from it. To produce single-walled CNTs (SWCNTs), catalyst particles smaller than 2 nanometers are required. Larger particles will produce multi-walled CNTs (MWCNTs).
The Substrate's Supporting Role
The substrate (often alumina or silica) is not just a passive holder. It prevents the catalyst nanoparticles from migrating and merging (sintering) at high synthesis temperatures. Maintaining small, discrete particles is essential for consistent and high-quality CNT growth.
The Impact of Temperature
Temperature is a critical operating parameter because it directly influences the catalyst's state. It must be high enough to keep the catalyst particle active for cracking the carbon source but not so high that it deactivates the particle or causes uncontrolled, amorphous carbon growth.
Understanding the Trade-offs
Selecting a catalyst system is an engineering decision that involves balancing competing priorities. There is no single "best" catalyst, only the most appropriate one for a given goal.
Catalyst Purity vs. Contamination
The most significant downside of this process is that the catalyst used to grow the CNTs becomes a major impurity in the final product. These residual metal particles must often be removed through intensive post-processing, typically with strong acids, which adds cost and can damage the CNTs.
Single-Walled vs. Multi-Walled Growth
The choice between SWCNTs and MWCNTs is a primary consideration. SWCNTs are prized for electronics due to their distinct electronic properties, but their growth requires extremely fine control over catalyst size. MWCNTs are easier to produce in bulk and are primarily used for mechanical reinforcement and conductivity enhancement in composites.
Cost vs. Performance
Iron is by far the cheapest and most common catalyst, making it ideal for the large-scale production of MWCNTs for applications like batteries and composites. Cobalt and various bimetallic alloys can offer more precise control over structure and are often favored in research and for high-performance electronic applications, but at a higher cost.
Aligning Catalyst Strategy with Your Goal
The optimal catalyst strategy depends entirely on the intended application of the carbon nanotubes.
- If your primary focus is bulk production for composites or batteries: Your best choice is a low-cost iron-based catalyst on a high-surface-area support, optimized for high yield and scalability.
- If your primary focus is high-performance electronics: You must prioritize precise catalyst size control, likely using cobalt or bimetallic alloys, to produce specific SWCNT diameters with minimal defects.
- If your primary focus is research and development: Your goal is to explore novel CNT properties by experimenting with different catalyst alloys, support materials, and growth conditions to tune structure.
Ultimately, mastering the catalyst is the key to unlocking the transformative potential of carbon nanotubes for any application.
Summary Table:
| Catalyst | Primary Use | Key Characteristic |
|---|---|---|
| Iron (Fe) | Bulk production (composites, batteries) | Low cost, high yield |
| Cobalt (Co) | High-performance electronics (SWCNTs) | Precise size control |
| Nickel (Ni) | General CNT synthesis | Balanced performance |
Ready to Optimize Your CNT Synthesis?
The right catalyst strategy is critical for achieving your specific carbon nanotube goals, whether for high-volume composites or precision electronics. KINTEK specializes in lab equipment and consumables, providing the tools and expertise to support your CNT research and production. Our solutions help you control catalyst parameters for superior nanotube quality and yield.
Contact us today to discuss how we can enhance your CNT growth process.
Visual Guide
Related Products
- Cylindrical Resonator MPCVD Machine System Reactor for Microwave Plasma Chemical Vapor Deposition and Lab Diamond Growth
- Custom PTFE Teflon Parts Manufacturer for PTFE Mesh F4 Sieve
- Zirconia Ceramic Gasket Insulating Engineering Advanced Fine Ceramics
- Shaking Incubators for Diverse Laboratory Applications
- Laboratory Disc Rotary Mixer for Efficient Sample Mixing and Homogenization
People Also Ask
- What machine is used to make lab-grown diamonds? Discover the HPHT & CVD Technologies
- What are the limitations of diamonds? Beyond the Myth of Perfection
- How does microwave plasma work? Unlock Precision Material Synthesis for Advanced Manufacturing
- Which lab grown diamond process is best? Focus on Quality, Not the Method
- What are the primary advantages of the CVD method for growing diamonds? Engineering High-Purity Gems and Components



















