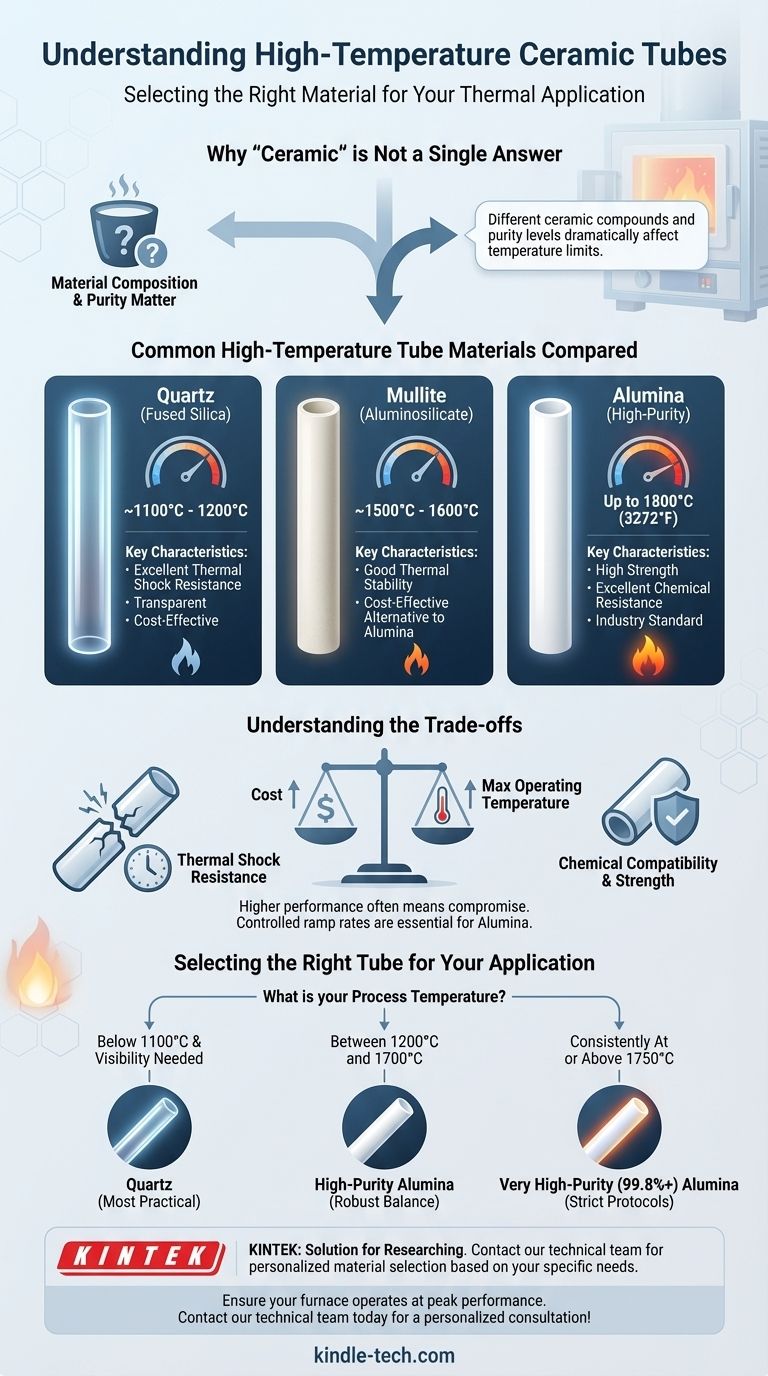
The maximum temperature for a ceramic tube is not a single value; it depends entirely on the specific ceramic material. While a transparent quartz tube is limited to around 1100°C, high-purity alumina ceramic tubes, often used in high-temperature furnaces, can operate continuously at temperatures up to 1800°C (3272°F).
The term 'ceramic tube' represents a wide category of materials, each with a distinct temperature limit. The central challenge is not finding one number, but selecting the specific material—like alumina or quartz—that precisely matches your required temperature, thermal shock resistance, and processing environment.

Why "Ceramic" Isn't a Single Answer
When selecting a tube for a high-temperature application, understanding the material composition is the most critical factor. The properties of a ceramic change dramatically based on its chemical makeup and purity.
The Role of Material Composition
Different ceramic compounds offer vastly different performance ceilings. A tube made of mullite will have a different maximum temperature than one made of pure alumina, even though both are considered "ceramics."
Purity Is Paramount
For any given ceramic, like alumina (Al₂O₃), higher purity directly translates to a higher service temperature. A 99.8% pure alumina tube can withstand significantly more heat and has better chemical resistance than a 95% pure alumina tube.
Common High-Temperature Tube Materials Compared
Choosing the right tube means comparing the most common options available for laboratory and industrial furnaces.
Quartz (Fused Silica)
Quartz is a type of ceramic glass known for its excellent thermal shock resistance and optical transparency. Its primary limitation is a relatively low maximum operating temperature, typically around 1100°C to 1200°C. It is unsuitable for the high-temperature ranges where alumina excels.
Alumina (Aluminum Oxide, Al₂O₃)
Alumina is the workhorse of high-temperature applications. It is strong, has excellent resistance to chemical attack, and performs well in both vacuum and oxidizing atmospheres.
High-purity alumina (99.5%+) tubes are the standard choice for furnaces operating in the 1400°C to 1800°C range. They offer the best combination of performance and cost for most high-heat processes.
Mullite (Aluminosilicate)
Mullite is another aluminosilicate ceramic that serves as a cost-effective alternative to high-purity alumina. It provides good thermal and mechanical stability up to approximately 1500°C to 1600°C, but it cannot reach the upper temperature limits of high-purity alumina.
Understanding the Trade-offs
Selecting a material is always a balance of competing factors. Higher performance in one area often means a compromise in another.
Temperature vs. Cost
There is a direct and steep correlation between maximum operating temperature and cost. Quartz is the most affordable, followed by mullite, with high-purity alumina being significantly more expensive. Specialized ceramics for even higher temperatures, like zirconia, carry an even greater premium.
Thermal Shock Resistance
Thermal shock—cracking caused by rapid temperature changes—is a primary cause of ceramic tube failure. Quartz is highly resistant to thermal shock. Alumina, while strong at high temperatures, is more brittle and susceptible to cracking if heated or cooled too quickly. A controlled ramp rate is essential for extending the life of an alumina tube.
Chemical Compatibility
The chemical environment inside the furnace is a critical consideration. While alumina is highly inert, certain reactive gases or materials at very high temperatures can degrade the tube over time. Always verify that your chosen tube material is compatible with your specific process chemicals.
Selecting the Right Tube for Your Application
Your final choice should be dictated by the specific demands of your work.
- If your process is below 1100°C and requires visibility: Quartz is your most practical and cost-effective choice.
- If you need to reach temperatures between 1200°C and 1700°C: A high-purity Alumina tube is the industry standard, offering a robust balance of performance and cost.
- If your process operates consistently at or above 1750°C: You must use a very high-purity (99.8%+) Alumina tube and adhere to strict heating and cooling protocols.
Understanding these material differences is the key to ensuring the reliability and success of your high-temperature work.
Summary Table:
| Material | Max Operating Temperature | Key Characteristics |
|---|---|---|
| Quartz (Fused Silica) | ~1100°C - 1200°C | Excellent thermal shock resistance, transparent, cost-effective |
| Mullite | ~1500°C - 1600°C | Good thermal stability, cost-effective alternative to alumina |
| Alumina (High-Purity) | Up to 1800°C | High strength, excellent chemical resistance, industry standard |
Selecting the correct high-temperature tube is critical for your process reliability and safety. KINTEK specializes in providing the right lab equipment and consumables for your specific needs. Our experts can help you choose the ideal ceramic tube material—whether quartz, mullite, or high-purity alumina—based on your target temperature, budget, and chemical environment.
Ensure your furnace operates at peak performance. Contact our technical team today for a personalized consultation!
Visual Guide
Related Products
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
- Vertical Laboratory Tube Furnace
- Multi-zone Laboratory Tube Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What tube is used for tubular furnace? Choose the Right Material for Temperature & Atmosphere
- What are the tubes in a furnace called? Understanding the Role of the Working Tube
- What are the advantages of using an alumina liner in a tube furnace for biomass combustion corrosion simulations?
- What is the primary advantage of using a tube furnace? Achieve Superior Temperature and Atmosphere Control
- Why is an Alumina Ceramic Tube Support Necessary for 1100°C Experiments? Ensure Data Accuracy and Chemical Inertness



















