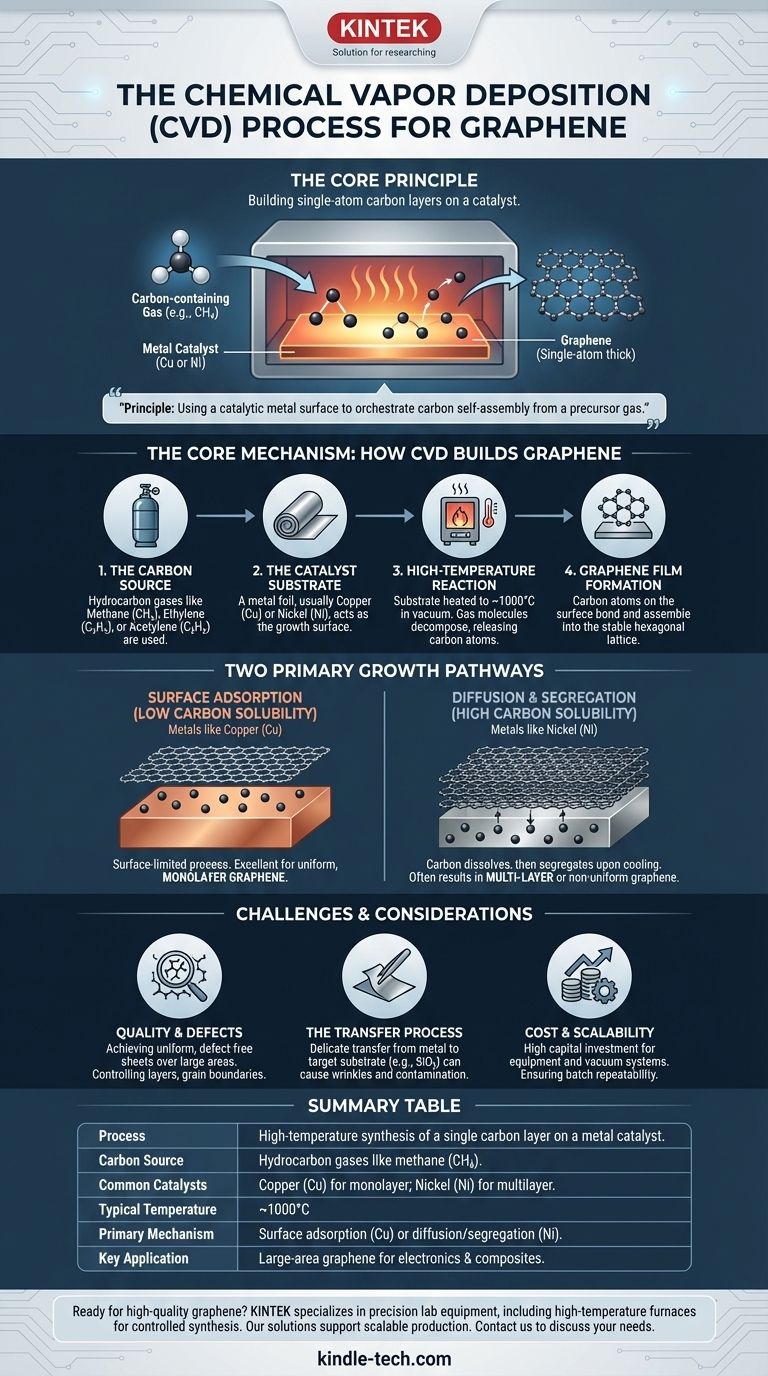
At its core, Chemical Vapor Deposition (CVD) for graphene is a synthesis process that builds a single-atom-thick layer of carbon on a catalyst surface. It works by introducing a carbon-containing gas, such as methane, into a high-temperature furnace containing a metal substrate, typically copper or nickel. The heat breaks down the gas, releasing carbon atoms that then assemble into the characteristic hexagonal lattice of graphene on the metal's surface.
The central principle of CVD is using a catalytic metal surface to orchestrate the self-assembly of carbon atoms from a precursor gas. This method is the leading strategy for producing high-quality, large-area graphene sheets suitable for industrial and electronic applications.

The Core Mechanism: How CVD Builds Graphene
The CVD process can be understood as a sequence of controlled steps occurring at the atomic level inside a specialized furnace.
The Carbon Source
The process begins with a carbon precursor. While solids and liquids can be used, this is most commonly a hydrocarbon gas.
Gases like methane (CH₄), ethylene (C₂H₄), or acetylene (C₂H₂) are standard choices because they can be precisely controlled and decompose cleanly at high temperatures.
The Catalyst Substrate
A metal foil, most often copper (Cu) or nickel (Ni), acts as the growth substrate or catalyst. This substrate is not just a passive surface; its chemical properties dictate the entire growth mechanism.
The High-Temperature Reaction
The metal substrate is heated to approximately 1000°C inside a vacuum chamber. The hydrocarbon gas is then introduced into the chamber.
At this extreme temperature, the gas molecules break apart, releasing individual carbon atoms onto the hot metal surface.
Graphene Film Formation
These free carbon atoms are highly mobile on the metal surface. They move around and bond with each other, naturally assembling into the stable, low-energy hexagonal lattice structure that defines graphene.
Two Primary Growth Pathways
The choice of metal substrate is critical because it determines exactly how the graphene film forms. There are two distinct mechanisms based on the metal's ability to dissolve carbon.
Surface Adsorption (Low Carbon Solubility)
Metals like copper have very low carbon solubility. This means carbon atoms cannot easily dissolve into the bulk of the metal.
The growth is therefore a surface-limited process. Carbon atoms land on the copper surface and stay there, forming a film. Once a complete single layer of graphene covers the surface, the catalytic action stops, making this an excellent method for producing uniform, monolayer graphene.
Diffusion and Segregation (High Carbon Solubility)
In contrast, metals like nickel have high carbon solubility. At high temperatures, carbon atoms readily dissolve and diffuse into the bulk of the nickel foil, like sugar dissolving in water.
When the system is cooled, the nickel's ability to hold carbon decreases sharply. This forces the dissolved carbon to precipitate back out onto the surface, where it crystallizes into graphene. This method can easily result in multi-layer or non-uniform graphene.
Understanding the Trade-offs and Challenges
While CVD is the most promising method for scalable graphene production, it is not without its complexities and limitations.
Quality and Defects
Achieving a perfectly uniform, defect-free sheet of graphene over a large area remains a significant challenge. Factors like layer number, grain boundaries (where different crystal domains meet), and twist angles between layers have a profound impact on the material's final electrical and mechanical properties.
The Transfer Process
Graphene grown on a metal foil is not immediately useful for most applications, such as electronics. It must be carefully transferred from the metal catalyst to a target substrate, like silicon dioxide or a flexible polymer.
This delicate transfer step is a major source of wrinkles, tears, and contamination that can degrade the graphene's quality.
Cost and Scalability
While described as "relatively inexpensive" compared to lab-scale methods like exfoliation, industrial CVD requires significant capital investment in high-temperature furnaces and vacuum equipment. Consistently controlling the process variables to ensure batch-to-batch repeatability is a key manufacturing hurdle.
Making the Right Choice for Your Goal
The optimal CVD approach depends entirely on the requirements of the final application.
- If your primary focus is high-performance electronics requiring uniform monolayer graphene: Your best approach is using a low-solubility substrate like copper to leverage its self-limiting growth mechanism.
- If your primary focus is applications where multi-layer graphene is acceptable or even beneficial (e.g., composites, coatings): Using a high-solubility substrate like nickel can be a more cost-effective and faster growth method.
Ultimately, mastering the CVD process is the key that unlocks graphene's potential to move from the laboratory to real-world technology.
Summary Table:
| Aspect | Key Detail |
|---|---|
| Process | High-temperature synthesis of a single carbon layer on a metal catalyst. |
| Carbon Source | Hydrocarbon gases like methane (CH₄). |
| Common Catalysts | Copper (Cu) for monolayer growth; Nickel (Ni) for multilayer growth. |
| Typical Temperature | ~1000°C |
| Primary Mechanism | Surface adsorption (Cu) or diffusion/segregation (Ni). |
| Key Application | Producing large-area graphene for electronics and composites. |
Ready to integrate high-quality graphene into your research or production? The CVD process is complex, but having the right equipment is the first step to success. KINTEK specializes in precision lab equipment and consumables, including the high-temperature furnaces essential for controlled graphene synthesis. Whether you are developing next-generation electronics or advanced composite materials, our solutions support reliable, scalable production. Contact our experts today via our Contact Form to discuss how we can meet your specific laboratory needs and help you achieve your materials science goals.
Visual Guide
Related Products
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
- Graphite Vacuum Furnace High Thermal Conductivity Film Graphitization Furnace
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
People Also Ask
- What are the different types of thin films? A Guide to Optical, Electrical, and Functional Coatings
- How does PECVD work? Enable Low-Temperature, High-Quality Thin Film Deposition
- How are thin films deposited? A Guide to PVD vs. CVD Methods for Your Application
- What is the difference between PECVD and CVD? Unlock the Right Thin-Film Deposition Method
- What are the steps of the CVD process? A Guide to Precision Thin Film Deposition



















