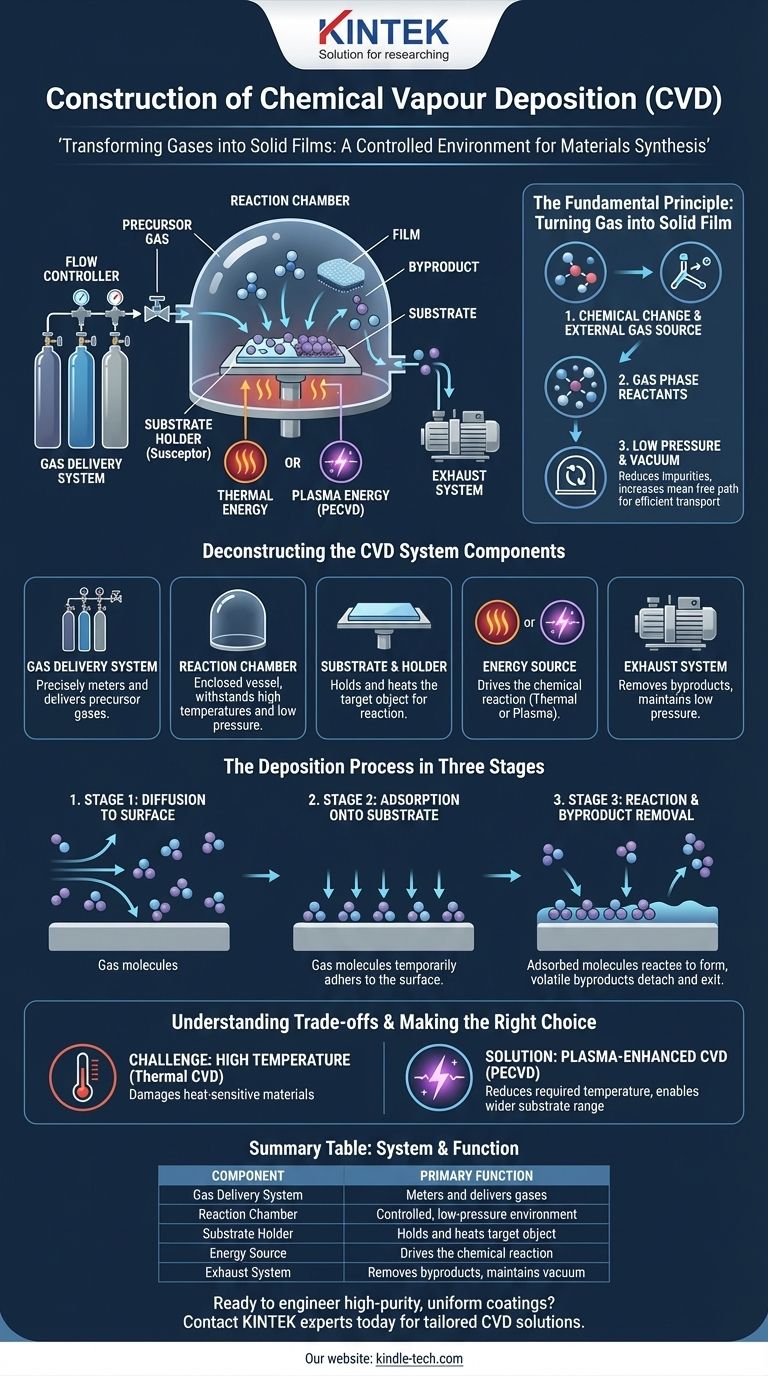
At its core, a Chemical Vapor Deposition (CVD) system is a controlled environment designed to transform gases into a solid film on a surface. The construction consists of a gas delivery system to introduce precursor chemicals, a reaction chamber held under low pressure, a substrate holder that is typically heated, an energy source to drive the chemical reaction, and an exhaust system to remove byproducts. This setup facilitates a process where gas molecules react on a target surface, building up a high-purity coating atom by atom.
The physical construction of a CVD system is less important than the process it enables. Its purpose is to create a highly controlled environment where gas-phase chemistry can be precisely manipulated to build a solid material with specific, desirable properties on a substrate.

The Fundamental Principle: Turning Gas into a Solid Film
Chemical Vapor Deposition is fundamentally a materials synthesis process. It works by flowing reactive gases (precursors) over a heated object (substrate) within a reaction chamber.
The Core Requirements
The process is defined by three essential characteristics. First, it involves a chemical change, such as a reaction or thermal decomposition. Second, all the material for the new film is supplied from an external source in the form of gas. Finally, the reactants must participate in the reaction as a gas phase.
The Role of Precursor Gases
Precursor gases are the chemical "building blocks" for the film. For example, to create a diamond film, a carbon-containing gas like methane is used along with hydrogen. These gases are carefully metered and fed into the reaction chamber.
The Importance of Low Pressure
CVD reactors are typically operated at low pressure or in a vacuum. This is critical for two reasons: it reduces impurity molecules that could contaminate the film and it increases the mean free path—the average distance a gas molecule travels before colliding with another. This ensures reactive gas molecules can efficiently reach and collide with the substrate.
Deconstructing the CVD System Components
While designs vary, all CVD systems are built around a few key functional components that manage the chemical process.
Gas Delivery System
This component consists of gas sources, valves, and mass flow controllers. It is responsible for precisely measuring and delivering the correct mixture of precursor and carrier gases into the reaction chamber.
The Reaction Chamber
This is the heart of the system, typically made of quartz or stainless steel. It is an enclosed vessel that contains the substrate and is designed to withstand high temperatures and maintain a low-pressure (vacuum) environment.
Substrate and Holder
The substrate is the material or object being coated. It is placed on a holder, often called a susceptor, which can be heated to the exact temperature required for the chemical reaction to occur on the substrate's surface.
The Energy Source
Energy is required to break down the precursor gases and drive the deposition reaction. This is supplied in two primary ways:
- Thermal Energy: In traditional CVD, the substrate is heated to very high temperatures (often 850-1100°C). This heat provides the energy for the reaction.
- Plasma Energy: In Plasma-Enhanced CVD (PECVD), an electromagnetic field (like microwaves) or an electric field is used to ionize the gas, creating a plasma. This highly reactive plasma allows the deposition to occur at much lower temperatures.
The Exhaust System
A vacuum pump is used to remove unreacted precursor gases and gaseous byproducts from the chamber. This maintains the low pressure and purges the system after the deposition is complete.
The Deposition Process in Three Stages
On a molecular level, the film's growth can be broken down into three distinct steps.
Stage 1: Diffusion to the Surface
After being introduced into the chamber, the reactant gas molecules move or diffuse from the main gas stream toward the substrate's surface.
Stage 2: Adsorption onto the Substrate
The gas molecules land on and are temporarily held on the substrate's surface, a process known as adsorption.
Stage 3: Reaction and Byproduct Removal
With sufficient energy from heat or plasma, the adsorbed molecules undergo a chemical reaction on the substrate surface. This forms the desired solid film and releases volatile byproducts, which then detach from the surface and are removed by the exhaust system.
Understanding the Trade-offs
While powerful, CVD technology involves critical limitations and design choices. Understanding these trade-offs is key to its successful application.
The Challenge of High Temperature
The primary limitation of conventional, thermally-driven CVD is the extremely high reaction temperature. Many potential substrate materials, such as polymers or certain electronics, cannot withstand this heat and would be damaged or destroyed.
The Solution: Plasma-Enhanced CVD (PECVD)
Using plasma to energize the gas, as in PECVD, dramatically reduces the required substrate temperature. This innovation makes it possible to deposit high-quality films on a much wider variety of heat-sensitive materials.
Control and Complexity
While some references describe the equipment as simple, achieving a uniform, high-purity film is complex. The final film's properties—its purity, crystal structure, and thickness—depend on a delicate balance of deposition parameters. These include temperature, pressure, gas flow rates, and chemical ratios, all of which must be precisely controlled.
Making the Right Choice for Your Goal
Your specific objective determines which aspect of the CVD process is most important.
- If your primary focus is depositing on heat-sensitive materials: Plasma-Enhanced CVD (PECVD) is the necessary approach due to its significantly lower operating temperatures.
- If your primary focus is creating highly pure, dense, and crystalline films: You must prioritize precise, repeatable control over all process parameters, especially temperature and gas flow.
- If your primary focus is coating complex 3D shapes: Leverage CVD's key advantage of providing excellent "wrap-around" properties for uniform, conformal coatings.
By understanding these core components and principles, you can effectively leverage CVD to engineer materials at the atomic level.
Summary Table:
| CVD System Component | Primary Function |
|---|---|
| Gas Delivery System | Precisely meters and delivers precursor gases into the chamber. |
| Reaction Chamber | Provides a controlled, low-pressure environment for the deposition reaction. |
| Substrate Holder (Susceptor) | Holds and heats the target object to the required temperature. |
| Energy Source (Heat/Plasma) | Drives the chemical reaction to deposit the solid film. |
| Exhaust System | Removes byproducts and maintains the chamber's low-pressure environment. |
Ready to engineer high-purity, uniform coatings for your substrates?
Whether your goal is depositing on heat-sensitive materials with PECVD or achieving highly crystalline films with precise thermal control, KINTEK's expertise in laboratory CVD equipment is your solution. We specialize in providing robust systems and consumables tailored to your specific research and production needs.
Contact our experts today to discuss how a KINTEK CVD system can advance your materials synthesis projects.
Visual Guide
Related Products
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
People Also Ask
- What are the different types of thin films? A Guide to Optical, Electrical, and Functional Coatings
- What is the process of vacuum vapor deposition? Mastering CVD and PVD Thin-Film Coating
- What is the vapor phase deposition technique? A Guide to PVD & CVD Thin-Film Coating Methods
- What is the difference between PECVD and CVD? Unlock the Right Thin-Film Deposition Method
- What are the methods of deposition? A Guide to PVD and CVD Thin-Film Techniques



















