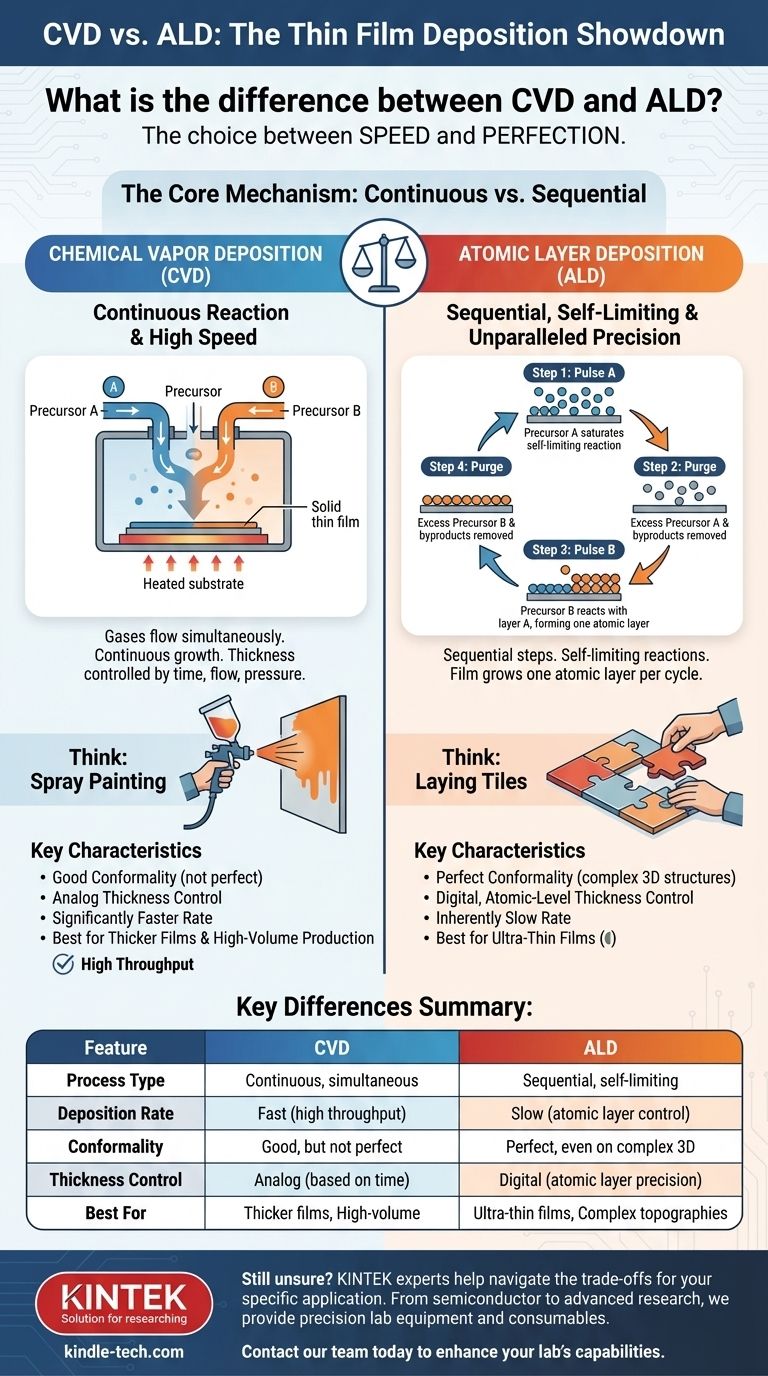
At its core, the difference between Chemical Vapor Deposition (CVD) and Atomic Layer Deposition (ALD) lies in how the chemical precursors are introduced to the substrate. CVD uses a continuous, simultaneous flow of reactive gases to grow a film, while ALD uses a sequential, self-limiting process that deposits the film one single atomic layer at a time. This fundamental difference in mechanism dictates every aspect of their performance, from speed to precision.
While both are chemical deposition techniques, the choice between them is a direct trade-off between speed and perfection. CVD offers high-speed deposition suitable for many applications, whereas ALD provides unparalleled precision and conformity at the cost of being significantly slower.

The Core Mechanism: Continuous vs. Sequential
The process mechanics are the single most important factor distinguishing CVD and ALD. This dictates the resulting film's properties.
How CVD Works: A Continuous Reaction
In a CVD process, one or more gaseous precursors are introduced into a reaction chamber simultaneously.
These gases react with each other and with the heated substrate surface, decomposing to form a solid thin film. The growth is continuous as long as the gases are flowing.
Think of it like spray painting: you continuously apply paint to a surface, and the thickness depends on how long you spray and how fast you move.
How ALD Works: A Self-Limiting Cycle
ALD breaks the deposition down into a cycle of two or more sequential steps.
First, a pulse of the first precursor gas is introduced. It reacts with the substrate surface until every available reaction site is occupied. This reaction is self-limiting; once the surface is saturated, no more material will deposit.
Next, the chamber is purged of any excess precursor. Then, a second precursor is pulsed in, reacting only with the first layer to complete a single atomic layer of the desired material. The cycle is repeated to build the film layer by atomic layer.
This is more like laying individual tiles. You place one set of tiles (precursor A), and they only fit in specific spots. Then you place the next set (precursor B) that only bonds to the first set, completing a perfect layer.
Key Differences in Performance and Quality
The mechanical differences between CVD and ALD lead to significant variations in the final product and its suitability for different applications.
Conformality: Coating Complex Shapes
ALD is perfectly conformal. Because the self-limiting reactions coat every available surface, ALD can produce a completely uniform film even inside deep trenches and complex, high-aspect-ratio 3D structures.
CVD has good, but not perfect, conformality. The continuous flow can lead to faster deposition at the opening of a feature than deep inside it, resulting in a non-uniform coating. It is vastly superior to line-of-sight techniques like PVD but cannot match ALD's perfection.
Thickness Control: Atomic vs. Bulk
ALD offers digital, atomic-level precision. Since each cycle deposits a known, fixed amount of material (typically a fraction of a monolayer), the final film thickness is controlled simply by counting the number of cycles.
CVD thickness control is analog. It depends on accurately managing gas flow rates, pressure, temperature, and deposition time. While highly controllable, it lacks the atomic-scale precision of ALD.
Deposition Rate: The Major Trade-off
CVD is significantly faster than ALD, often by one or two orders of magnitude. Its continuous growth process is well-suited for depositing thicker films (from hundreds of nanometers to microns) or for high-volume manufacturing.
ALD is inherently slow. The need to pulse and purge gases for each individual atomic layer makes it impractical for thick films. It is reserved for applications where ultra-thin films (typically under 100 nm) and perfect control are required.
Understanding the Practical Trade-offs
Choosing between CVD and ALD is not about which is "better," but which is the correct tool for your specific engineering goal. The decision always involves balancing competing priorities.
The Speed vs. Precision Dilemma
This is the central trade-off. If your application can tolerate minor imperfections in thickness or conformity but requires high throughput, CVD is the logical choice.
If your device's performance absolutely depends on a perfectly uniform, pinhole-free film with precise thickness, especially on a complex topography, then ALD is the only option, and you must accept the slower deposition time.
Temperature and Substrate Sensitivity
The challenges of thin film deposition often include temperature limitations. ALD processes can frequently be run at lower temperatures than many traditional CVD processes.
This makes ALD highly suitable for depositing films onto temperature-sensitive substrates, such as polymers or pre-processed semiconductor wafers that cannot withstand high thermal loads.
Cost and Complexity
CVD systems are generally simpler and less expensive. The technology is mature and widely used for large-scale production.
ALD systems require highly precise, fast-acting valves and control systems to manage the pulse-and-purge cycles, which can increase equipment complexity and cost. Precursor chemistry for ALD can also be more specialized and expensive.
Making the Right Choice for Your Goal
Your application's specific requirements will point you to the correct deposition method.
- If your primary focus is ultimate precision and perfect coverage on complex 3D structures (e.g., advanced transistors, MEMS, nano-coatings): ALD is the definitive choice for its unmatched conformity and atomic-level control.
- If your primary focus is high-throughput production of quality films on simpler surfaces (e.g., protective tool coatings, optics, standard semiconductor layers): CVD offers an excellent balance of speed, film quality, and cost-effectiveness.
- If your primary focus is depositing a material that requires very low temperatures and high density (e.g., sensitive electronics, flexible devices): ALD's lower temperature window and high-quality film growth make it a superior option.
Ultimately, your choice is a strategic decision that balances the demand for perfection against the realities of production efficiency and cost.
Summary Table:
| Feature | Chemical Vapor Deposition (CVD) | Atomic Layer Deposition (ALD) |
|---|---|---|
| Process Type | Continuous, simultaneous gas flow | Sequential, self-limiting cycle |
| Deposition Rate | Fast (high throughput) | Slow (atomic layer control) |
| Conformality | Good, but not perfect | Perfect, even on complex 3D structures |
| Thickness Control | Analog (based on time/flow) | Digital (atomic layer precision) |
| Best For | Thicker films, high-volume production | Ultra-thin films, complex topographies |
Still unsure whether CVD or ALD is right for your lab's thin film deposition needs?
At KINTEK, we specialize in providing precision lab equipment and consumables for all your deposition requirements. Our experts can help you navigate the trade-offs between speed and precision to select the ideal solution for your specific application—whether you're working on semiconductor fabrication, MEMS, protective coatings, or advanced research.
Let us help you achieve perfect results. Contact our team today to discuss your project and discover how KINTEK's solutions can enhance your laboratory's capabilities and efficiency.
Visual Guide
Related Products
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- Multi Heating Zones CVD Tube Furnace Machine Chemical Vapor Deposition Chamber System Equipment
People Also Ask
- What are deposition systems for the semiconductor industry? The Master Builders of Modern Microchips
- Which deposition technique is used for metals? A Guide to PVD Methods for Thin Film Coating
- Can polymers be deposited using CVD processes? Yes, for High-Purity, Conformal Films
- What is deposition gas examples? Discover Key Gases That Turn Directly to Solid
- What is sputtering for thin film deposition? A Guide to High-Performance Coating Technology
- Why do we need vacuum for deposition of thin film? Ensure Purity and Control in Your Lab
- What function does a Chemical Vapor Deposition (CVD) system serve? Essential Tools for High-Heat Composites
- Are the important thin film deposition methods? PVD vs. CVD Explained for Your Application



















