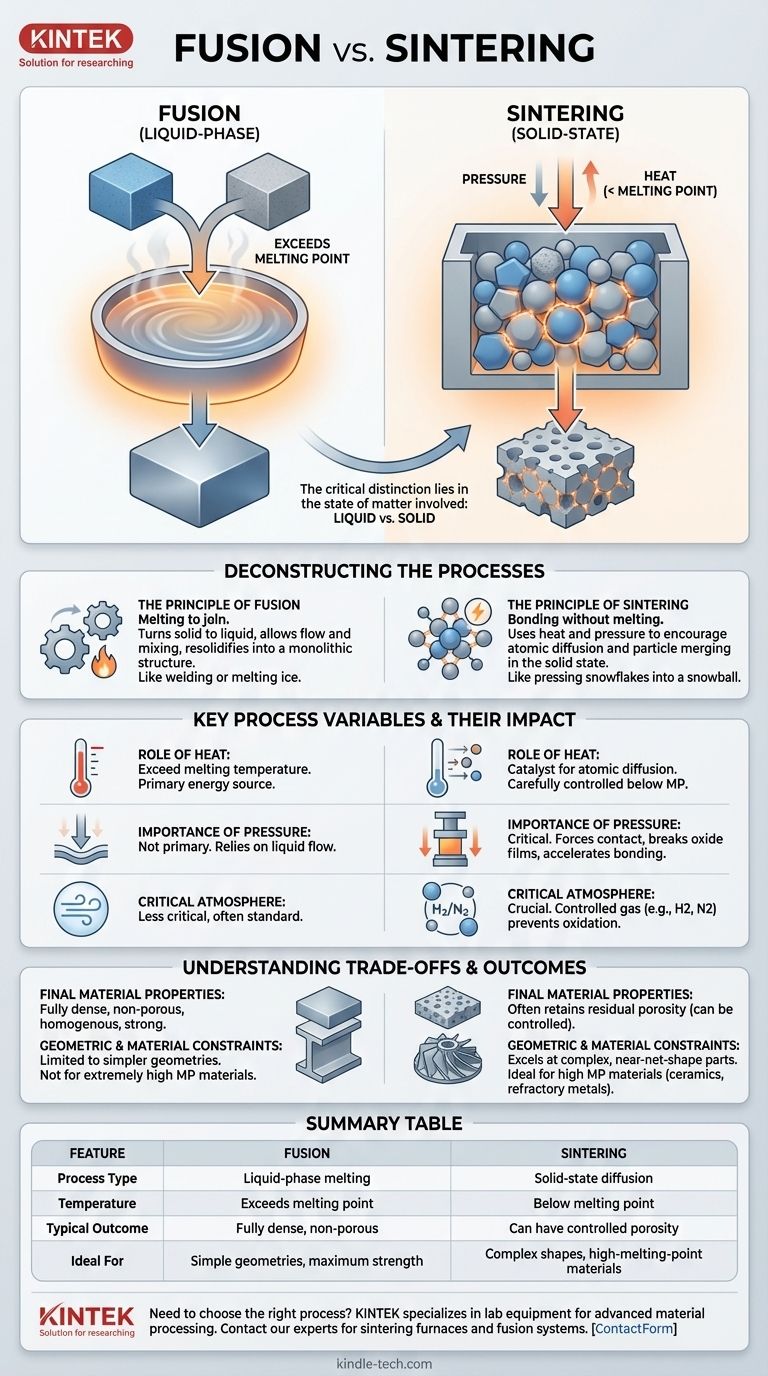
At its core, fusion is the process of joining materials by melting them together, while sintering joins them without reaching their melting point. Fusion creates a bond by turning solid material into a liquid and letting it resolidify, effectively creating a single, continuous piece. Sintering, however, is a solid-state process that uses heat and pressure to encourage individual particles to bond and densify.
The critical distinction lies in the state of matter involved. Fusion relies on a complete transition to a liquid phase to create a bond, whereas sintering uses heat and pressure to force solid particles to merge at an atomic level, never fully melting.

Deconstructing the Processes: Melting vs. Bonding
Understanding the mechanism behind each process is key to appreciating their distinct applications and outcomes. They represent two fundamentally different approaches to creating a solid mass.
The Principle of Fusion
Fusion is the more intuitive process. Think of welding two steel plates or melting two ice cubes together.
The goal is to apply enough energy, almost always heat, to exceed the material's melting point. This phase change allows the materials to flow together and mix on a macroscopic level, creating a uniform, monolithic structure once cooled.
The Principle of Sintering
Sintering is a more complex phenomenon that occurs entirely in the solid state. Imagine pressing a handful of snowflakes together to form a denser snowball—they bond without ever turning to water.
The process uses heat to energize the atoms within a mass of powder. This energy, applied at a temperature below the melting point, allows atoms to diffuse across the boundaries of adjacent particles, effectively merging them.
Key Process Variables and Their Impact
The specific parameters of heat, pressure, and atmosphere are what control the outcome of each process.
The Role of Heat
In fusion, the function of heat is straightforward: to exceed the melting temperature.
In sintering, heat is a catalyst for atomic diffusion. The temperature must be carefully controlled—high enough to allow atoms to move, but low enough to prevent melting, which would destroy the part's shape.
The Importance of Pressure
Pressure is not always a primary factor in fusion, which relies on the material's ability to flow in its liquid state.
For sintering, pressure is often critical. As seen in processes like hot pressing, pressure forces particles into intimate contact, which helps break down surface oxide films and accelerates the atomic bonding process.
The Critical Atmosphere
The surrounding environment plays a crucial role, especially for reactive materials.
During sintering, materials like metals, nitrides, or carbides often require a specific gas atmosphere, such as hydrogen or nitrogen. This controlled environment prevents oxidation and other unwanted chemical reactions that would inhibit proper bonding and densification.
Understanding the Trade-offs and Outcomes
The choice between fusion and sintering has direct consequences for the final product's properties and manufacturability.
Final Material Properties
Fusion processes typically result in a fully dense, non-porous material that is homogenous and strong, similar to the original cast material.
Sintered parts, on the other hand, often retain a small amount of residual porosity. While this can sometimes reduce ultimate strength, it can also be a desired feature for applications like self-lubricating bearings or filters.
Geometric and Material Constraints
Fusion is often limited to simpler geometries and is not suitable for materials with extremely high melting points, as reaching those temperatures can be impractical and costly.
Sintering excels at producing complex, near-net-shape parts from materials with very high melting points, such as ceramics and refractory metals. It is the foundation of powder metallurgy and many forms of metal and ceramic 3D printing.
Making the Right Choice for Your Application
Selecting the correct process depends entirely on the material you are using and the properties you need in the final component.
- If your primary focus is maximum density and strength in a simple geometry: Fusion processes like welding or casting are often the most direct path.
- If your primary focus is creating complex shapes from high-melting-point materials like ceramics or tungsten: Sintering is the industry-standard and often the only viable method.
- If your primary focus is producing parts with controlled porosity for filters or bearings: Sintering provides unique and essential control over the final part density.
Understanding this fundamental difference between liquid-phase melting and solid-state bonding is the key to mastering material processing.
Summary Table:
| Feature | Fusion | Sintering |
|---|---|---|
| Process Type | Liquid-phase melting | Solid-state diffusion |
| Temperature | Exceeds melting point | Below melting point |
| Typical Outcome | Fully dense, non-porous | Can have controlled porosity |
| Ideal For | Simple geometries, maximum strength | Complex shapes, high-melting-point materials |
Need to choose the right process for your materials? KINTEK specializes in lab equipment and consumables for advanced material processing. Our expertise in sintering furnaces and fusion systems can help you achieve precise results, whether you're working with high-performance ceramics or complex metal alloys. Contact our experts today to discuss your application and find the perfect solution for your laboratory needs.
Visual Guide
Related Products
- 1700℃ Muffle Oven Furnace for Laboratory
- 1800℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What is the difference between melting and sintering temperatures? A Guide to Material Processing Methods
- What is melt loss? The Ultimate Guide to Reducing Metal Loss in High-Temp Processing
- What is done by ashing in muffle furnace? A Guide to Precise Inorganic Content Analysis
- What is a laboratory furnace called? A Guide to Muffle and Tube Furnaces
- What is the construction and working of a muffle furnace? A Guide to Precise, Contaminant-Free Heating



















