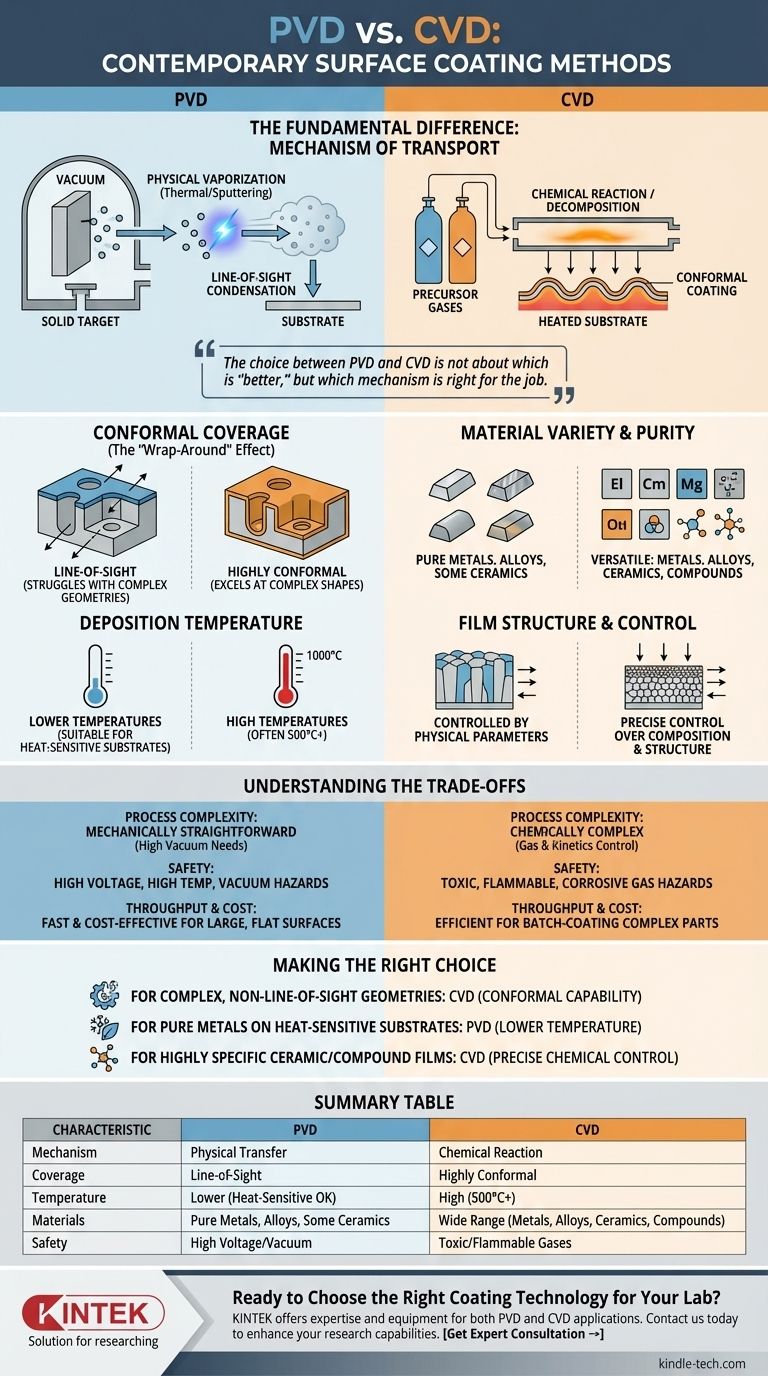
The fundamental difference between Physical Vapor Deposition (PVD) and Chemical Vapor Deposition (CVD) lies in how the coating material is transported to the substrate. PVD is a mechanical process where a solid or liquid source material is physically vaporized and then condenses onto the part. In contrast, CVD is a chemical process where precursor gases react or decompose on the substrate's surface to form the coating.
The choice between PVD and CVD is not about which is "better," but which mechanism is right for the job. PVD is a physical, line-of-sight process ideal for depositing pure materials onto simpler geometries, while CVD uses chemical reactions to create highly conformal coatings on even the most complex surfaces.

The Core Mechanism: How Each Process Works
To select the right technology, you must first understand the fundamental difference in how the film is formed. One is a process of physical transfer, the other of chemical creation.
Physical Vapor Deposition (PVD): A Physical Transfer
In PVD, the coating material starts as a solid target. This target is transformed into a vapor through purely physical means inside a high-vacuum chamber.
The two primary methods are thermal evaporation, which uses high temperatures to boil off atoms from the target, and sputtering, which uses a high-energy plasma to bombard the target and physically dislodge atoms.
These vaporized atoms then travel in a straight line—a "line-of-sight" path—until they strike the substrate and condense, forming the solid film.
Chemical Vapor Deposition (CVD): A Chemical Reaction
CVD begins not with a solid target, but with one or more volatile precursor gases introduced into a reaction chamber.
These gases decompose or react with each other on the surface of a heated substrate. This chemical reaction is what forms the solid coating material directly on the part.
Because the deposition is driven by a chemical reaction in a gaseous environment, the material can deposit uniformly on all exposed surfaces, regardless of their orientation to the gas source.
Comparing Key Coating Characteristics
The difference in mechanism directly dictates the properties of the final coating and the types of parts that can be processed.
Conformal Coverage (The "Wrap-Around" Effect)
CVD excels at producing highly conformal coatings. Its gas-phase nature allows it to uniformly coat intricate, complex shapes and internal surfaces with ease.
PVD, being a line-of-sight process, struggles with this. It requires complex fixtures and part rotation to achieve coverage on non-flat geometries, and coating inside deep holes or channels is often impossible.
Material Variety and Purity
CVD is exceptionally versatile, capable of depositing a wide range of materials including metals, multi-component alloys, and highly pure ceramic or compound layers by simply adjusting the precursor gases.
PVD is also versatile but is most often used for depositing pure metals, specific alloys, and some ceramic compounds. The composition of the film is directly tied to the composition of the physical target.
Deposition Temperature and Substrate Impact
Traditional CVD processes often require very high temperatures (many hundreds or even over 1000°C) to drive the necessary chemical reactions. This limits the types of substrate materials that can be coated without being damaged or distorted.
While some PVD processes use heat, many, like sputtering, can be performed at much lower temperatures. This makes PVD suitable for coating heat-sensitive materials like plastics, or finished components where high heat would alter their properties.
Film Structure and Control
CVD allows for fine control over the film's chemical composition, crystal structure, and grain size by precisely managing gas flow rates, pressure, and temperature.
In PVD, film properties are controlled by physical parameters like the deposition rate, plasma energy, and chamber pressure.
Understanding the Trade-offs
Neither method is a universal solution. Each comes with its own set of operational considerations and limitations.
Process Complexity
CVD processes can be chemically complex. Success depends on precise control over gas mixtures and reaction kinetics, and managing byproducts.
PVD is mechanically straightforward in concept, but achieving high-quality films requires rigorous control over vacuum levels, power sources, and the physical setup of the chamber.
Safety and Environmental Concerns
CVD often involves precursor gases that are toxic, flammable, or corrosive, necessitating sophisticated handling and safety systems.
PVD processes are generally considered cleaner from a chemical standpoint. The primary hazards are related to the high voltages, high temperatures, and high-vacuum environments used.
Throughput and Cost
The economics of each process are highly application-dependent. CVD can be very efficient for batch-coating large numbers of complex parts due to its excellent conformal coverage.
PVD can be extremely fast and cost-effective for coating large, flat surfaces in an inline system, but becomes less efficient for complex geometries that require manipulation.
Making the Right Choice for Your Application
Your decision should be driven by the geometry of your part and the desired properties of the final film.
- If your primary focus is coating complex, non-line-of-sight geometries: CVD is the superior choice due to its inherent chemical "wrap-around" capability.
- If your primary focus is depositing pure metals or common alloys onto heat-sensitive substrates: PVD, particularly sputtering, offers a lower-temperature and highly controllable solution.
- If your primary focus is creating highly specific ceramic, compound, or high-purity films: CVD provides unparalleled control over the film's final chemical makeup through precise gas management.
Understanding the fundamental difference between physical transfer and chemical creation is the key to selecting the optimal coating technology for your specific engineering challenge.
Summary Table:
| Characteristic | PVD (Physical Vapor Deposition) | CVD (Chemical Vapor Deposition) |
|---|---|---|
| Mechanism | Physical transfer of solid/liquid material | Chemical reaction of precursor gases |
| Coverage | Line-of-sight (limited complex geometries) | Highly conformal (wraps around complex shapes) |
| Temperature | Lower temperatures (suitable for heat-sensitive substrates) | High temperatures (often 500°C+) |
| Materials | Pure metals, alloys, some ceramics | Wide range: metals, alloys, ceramics, compounds |
| Safety | High voltage/vacuum hazards | Toxic, flammable, corrosive gas hazards |
Ready to Choose the Right Coating Technology for Your Lab?
Whether you need PVD for heat-sensitive substrates or CVD for complex geometries, KINTEK has the expertise and equipment to support your surface coating requirements. Our team specializes in helping laboratories select and implement the optimal deposition method for their specific applications.
Contact us today to discuss your project needs and discover how KINTEK's lab equipment solutions can enhance your research capabilities.
Visual Guide
Related Products
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- HFCVD Machine System Equipment for Drawing Die Nano-Diamond Coating
- Vacuum Hot Press Furnace Machine for Lamination and Heating
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- Vertical Laboratory Tube Furnace
People Also Ask
- What are different types of thin films? A Guide to Function, Material, and Deposition Methods
- Why does a PECVD vacuum system require both a rotary vane and turbo pump? Ensure High-Purity Coatings
- Why is a Matching Network Indispensable in RF-PECVD for Siloxane Films? Ensure Stable Plasma and Uniform Deposition
- Can plasma enhanced CVD deposit metals? Why PECVD is rarely used for metal deposition
- How are thin films deposited? A Guide to PVD vs. CVD Methods for Your Application



















