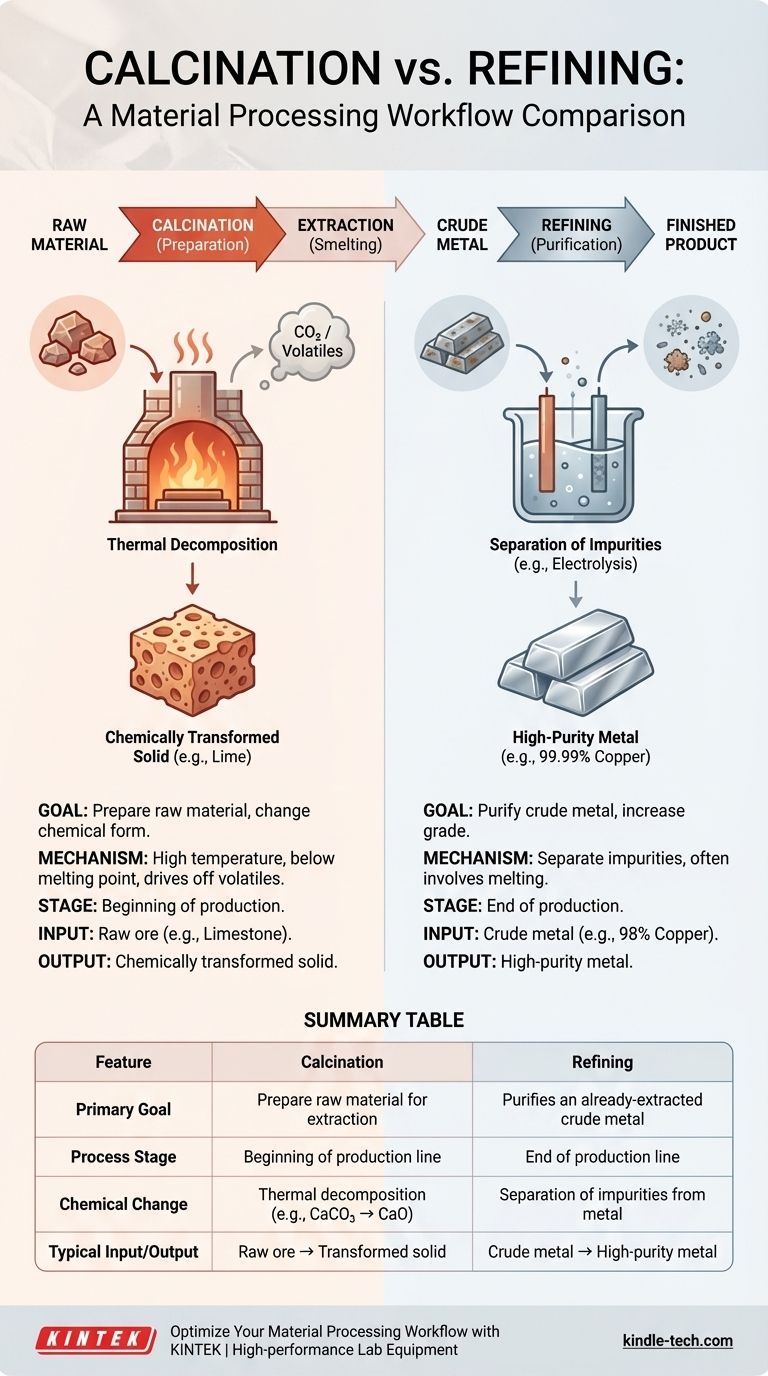
At its core, refining and calcination are two distinct stages in material processing with opposite goals. Calcination is a preparatory, high-temperature process used to break down raw materials like ore into a more reactive state, whereas refining is a final purification process used to remove lingering impurities from an already-extracted crude metal.
The simplest way to distinguish them is to consider their place in the production line: Calcination prepares the raw material at the beginning of the process, while refining perfects the near-finished product at the end.

What is Calcination? A Preparatory Transformation
Calcination is a thermal treatment process applied to ores and other solid materials to bring about a chemical change. Its primary purpose is to prepare the material for a subsequent step, like smelting.
The Core Mechanism: Thermal Decomposition
The defining feature of calcination is heating a solid to a high temperature, but below its melting point. This is done in the absence of, or with limited, air.
The intense heat breaks chemical bonds, causing the material to decompose. This typically drives off a volatile component, such as carbon dioxide (CO2) or chemically bound water (hydrates).
A classic example is heating limestone (calcium carbonate, CaCO3) to produce lime (calcium oxide, CaO) and carbon dioxide gas. The solid CaCO3 is transformed into solid CaO, a new chemical substance.
The Primary Goal: Changing Chemical Form
The goal of calcination is not purification in the traditional sense. It is about changing the ore into a more suitable chemical form for metal extraction.
For instance, converting a metal carbonate ore into a metal oxide via calcination makes the subsequent extraction of the metal (often through smelting) far more energy-efficient.
What is Refining? A Final Purification
Refining refers to a set of processes used to purify an impure, crude metal that has already been extracted from its ore. The goal is to increase the grade, or purity, of the metal.
The Core Mechanism: Separating Impurities
Refining processes work on material that is already metallic, but contains small percentages of other elements. Unlike calcination, refining often involves melting the metal.
Techniques vary widely depending on the metal and the impurities. Examples include:
- Electrolytic Refining: Used for copper, this process uses an electric current to dissolve an impure anode and deposit ultra-pure metal onto a cathode.
- Fractional Distillation: Used for metals with low boiling points like zinc, this separates metals based on their different boiling points.
- Liquation: Used when impurities have a higher melting point than the metal. The mixture is heated just enough to melt the desired metal, allowing it to flow away from the solid impurities.
The Primary Goal: Achieving High Purity
The sole purpose of refining is to remove the last remaining contaminants to meet the stringent specifications required for commercial use.
A smelter might produce copper that is 98% pure. For use in electrical wiring, that copper must be refined to 99.99% purity to ensure high conductivity. That final step is refining.
Understanding the Trade-offs and Sequence
These processes are not interchangeable; they are sequential steps in a larger workflow, and using one where the other is needed would be ineffective and costly.
Why You Can't Refine Raw Ore
Refining processes are designed to handle materials that are already in a high-concentration metallic form.
Applying an energy-intensive process like electrolysis to a vast quantity of raw ore, which might contain only 2% metal, would be economically and technically impossible. First, you must concentrate the ore and extract the crude metal.
Why Calcination Doesn't Produce Pure Metal
Calcination only changes the chemical compound; it does not separate the desired element from the rest of the ore's rock and gangue (the worthless material).
The lime produced from calcining limestone is calcium oxide, not pure calcium. The metal is still chemically bonded to oxygen and mixed with other minerals, requiring further processing to be liberated.
Making the Right Choice for Your Goal
Understanding the role of each process is key to understanding material science and metallurgy.
- If your primary focus is preparing a carbonate or hydrated ore for a smelter: You will use calcination to convert the ore into an oxide, making it easier to reduce to a metal.
- If your primary focus is producing 99.99% pure copper for electronics: You will use electrolytic refining on crude copper that has already been extracted from its ore.
- If your primary focus is understanding the full journey from rock to product: You recognize calcination as an early-stage chemical conversion and refining as a final-stage purification.
Each step in the journey from raw earth to finished material serves a specific and critical purpose.
Summary Table:
| Feature | Calcination | Refining |
|---|---|---|
| Primary Goal | Prepares raw material for extraction | Purifies an already-extracted crude metal |
| Process Stage | Beginning of the production line | End of the production line |
| Chemical Change | Thermal decomposition (e.g., CaCO₃ → CaO) | Separation of impurities from the metal |
| Typical Input | Raw ore (e.g., limestone) | Crude, impure metal (e.g., 98% copper) |
| Typical Output | Chemically transformed solid (e.g., lime) | High-purity metal (e.g., 99.99% copper) |
Optimize Your Material Processing Workflow with KINTEK
Understanding the precise role of each thermal process is crucial for efficiency and product quality in your laboratory or production facility. Whether you are preparing materials with calcination or achieving ultra-high purity through refining, having the right equipment is fundamental.
KINTEK specializes in high-performance lab equipment for all stages of material processing. Our range of furnaces and reactors are designed to deliver the precise temperature control and atmospheric conditions required for both calcination and specialized refining techniques.
Let us help you enhance your process:
- Achieve consistent results with reliable thermal processing equipment.
- Improve efficiency with solutions tailored to your specific material and purity goals.
- Access expert support from a team dedicated to serving the needs of laboratories and research facilities.
Ready to perfect your process from preparation to purification? Contact our experts today to discuss how KINTEK's lab equipment and consumables can meet your specific challenges.
Visual Guide
Related Products
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Laboratory Vacuum Tilt Rotary Tube Furnace Rotating Tube Furnace
- Rotary Tube Furnace Split Multi Heating Zone Rotating Tube Furnace
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
People Also Ask
- What are the components of a rotary kiln? A Guide to the Core Systems and Parts
- What biomass is used in pyrolysis? Selecting the Optimal Feedstock for Your Goals
- What is the meaning of rotary furnace? Achieve Superior Uniformity in Continuous Heat Treatment
- What are the three types of pyrolysis process? Slow, Fast, and Conventional Explained
- How does pyrolysis work without oxygen? Transform Waste into Valuable Products
- What is the aim of calcination and roasting? Master Ore Preparation for Metal Extraction
- What is a rotary tube furnace? Achieve Superior Uniformity for Powders and Granules
- What is the limitations of pyrolysis? Key Economic and Technical Challenges to Consider



















