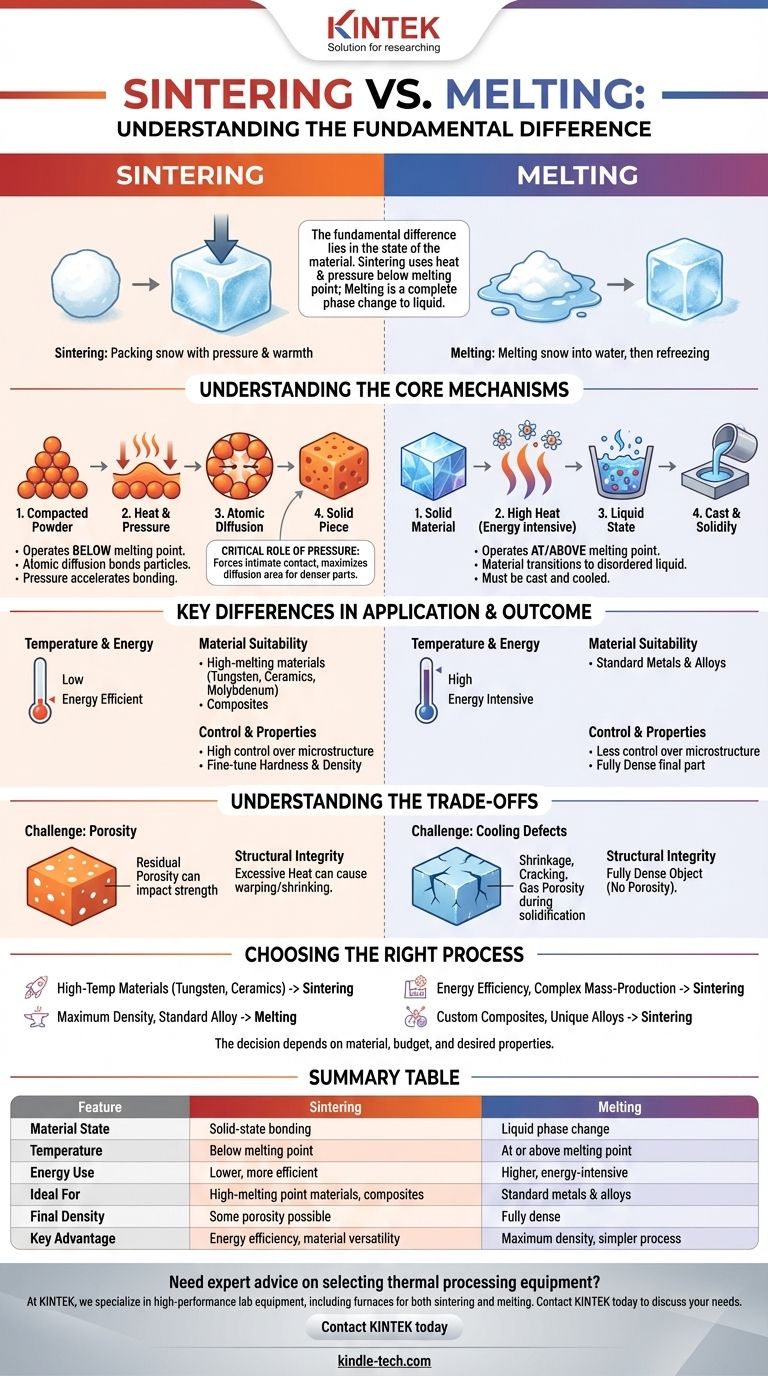
The fundamental difference between sintering and melting lies in the state of the material during the process. Melting is a complete phase change where a material is heated until it turns from a solid into a liquid. Sintering, in contrast, uses a combination of heat and pressure to bond material particles together at a molecular level without ever reaching the liquid state.
Think of it this way: melting is like making a single, solid ice cube by melting down a pile of snow and refreezing it. Sintering is like packing that snow into a dense, solid block by applying immense pressure and just enough warmth to fuse the individual snowflakes together where they touch.

Understanding the Core Mechanisms
To grasp the practical implications of these two processes, it's essential to understand how each one fundamentally works to create a solid object from a base material.
How Melting Works
Melting relies exclusively on thermal energy. As you heat a material, its atoms vibrate more intensely until they break free from their fixed, crystalline structure.
The material transitions into a disordered liquid state. To form a final part, this liquid must then be cast into a mold and cooled, allowing it to solidify again.
How Sintering Works
Sintering operates at temperatures below the material's melting point. The process begins with a compacted powder of the desired material.
Heat provides the energy for a phenomenon called atomic diffusion. Atoms from individual particles migrate across the boundaries to the adjacent particles, creating strong metallic bonds and fusing the powder into a coherent, solid piece.
The Critical Role of Pressure
While heat drives atomic diffusion, pressure is often a critical catalyst in sintering.
Applying external pressure forces the material particles into intimate contact. This maximizes the surface area where diffusion can occur, accelerates the bonding process, and helps create a denser final part.
Key Differences in Application and Outcome
The distinction between a solid-state process (sintering) and a liquid-state process (melting) leads to significant differences in energy use, material compatibility, and the properties of the final product.
Temperature and Energy Consumption
Melting is an energy-intensive process. It requires heating a material not only to its melting point but also providing the additional energy (latent heat of fusion) needed for the phase change.
Sintering is significantly more energy-efficient. By operating at lower temperatures, it consumes far less energy, making it a more economical choice for many industrial applications.
Material Suitability
Melting is straightforward for many standard metals and alloys. However, it becomes impractical or extremely expensive for materials with exceptionally high melting points.
Sintering excels in this area. It is the preferred method for fabricating parts from materials like tungsten, molybdenum, and advanced ceramics, which are too difficult to melt. It also enables the creation of unique composites by combining powders of different materials.
Control and Final Properties
The sintering process offers a high degree of control over the final part's microstructure. By carefully managing temperature, pressure, and time, manufacturers can fine-tune properties like hardness and density.
Melting and casting produce a fully dense part but offer less control over the fine-grained microstructure, which is formed during the cooling and solidification phase.
Understanding the Trade-offs
Neither process is universally superior. The optimal choice depends on balancing the inherent advantages and disadvantages of each method.
The Challenge of Porosity
Sintering almost always leaves some degree of residual porosity in the final part. These microscopic voids between the original powder particles can impact the overall strength and density.
Melting, by its nature, creates a fully dense object, as the liquid material fills all available space in a mold before solidifying.
Structural Integrity
Excessive heat during sintering is a critical failure point. If the temperature gets too high and approaches the melting point, it can cause the part to warp, shrink unevenly, or lose its intended shape.
Imperfections in melted parts typically arise during cooling. Issues like shrinkage, cracking, or gas porosity can occur as the liquid metal solidifies.
Process Complexity
While conceptually simple, high-performance sintering can be a complex process. It requires precise control over the furnace atmosphere, temperature ramps, and pressure application to achieve consistent results.
Melting is often a simpler, more direct process, but it may require significant post-processing (like machining) to achieve the final desired shape and surface finish.
Choosing the Right Process for Your Goal
The decision to use sintering or melting is driven entirely by your material, budget, and the desired properties of the final component.
- If your primary focus is creating parts from high-temperature materials like tungsten or ceramics: Sintering is the practical and often only viable choice.
- If your primary focus is achieving maximum density and a non-porous structure for a standard alloy: Melting and casting is generally the more direct path.
- If your primary focus is energy efficiency and mass-producing complex, near-net-shape parts: Sintering, a cornerstone of powder metallurgy, offers significant cost advantages.
- If your primary focus is creating custom metal matrix composites or unique alloys: Sintering allows you to combine materials that could not be mixed in a liquid state.
Ultimately, understanding that melting builds from a liquid while sintering builds from a solid is the key to making an informed manufacturing decision.
Summary Table:
| Feature | Sintering | Melting |
|---|---|---|
| Material State | Solid-state bonding | Liquid phase change |
| Temperature | Below melting point | At or above melting point |
| Energy Use | Lower, more efficient | Higher, energy-intensive |
| Ideal For | High-melting point materials (tungsten, ceramics), composites | Standard metals and alloys |
| Final Density | Some porosity possible | Fully dense |
| Key Advantage | Energy efficiency, material versatility | Maximum density, simpler process for standard metals |
Need expert advice on selecting the right thermal processing equipment for your lab?
At KINTEK, we specialize in high-performance lab equipment, including furnaces for both sintering and melting applications. Whether you're working with advanced ceramics, high-temperature metals, or standard alloys, our solutions are designed to deliver precision, efficiency, and reliability.
Let our experts help you optimize your process for superior results. Contact KINTEK today to discuss your specific needs and discover how our equipment can enhance your laboratory's capabilities.
Visual Guide
Related Products
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
- Vacuum Heat Treat and Sintering Furnace with 9MPa Air Pressure
- Spark Plasma Sintering Furnace SPS Furnace
- Dental Porcelain Zirconia Sintering Ceramic Furnace Chairside with Transformer
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
People Also Ask
- What is the use of a digital muffle furnace? Achieve Contamination-Free High-Temperature Processing
- How do you calibrate a muffle furnace? Achieve Precise Temperature Control for Your Lab
- What is the purpose of using a high-temperature furnace for SiC powder pre-treatment? Enhance Composite Bond Strength
- What role does a laboratory furnace with an observation window play in refractoriness testing? Real-time Data Accuracy
- How long does heating take on a muffle furnace? Unlock the Key Factors for Your Lab's Efficiency
- What is a muffle furnace used for in microbiology? Essential for Depyrogenation and Ashing
- What is the principle of sintering furnace? Harnessing Heat and Atmosphere for Material Transformation
- What is the meaning of ash furnace? Uncover Material Composition with Precision Ashing



















