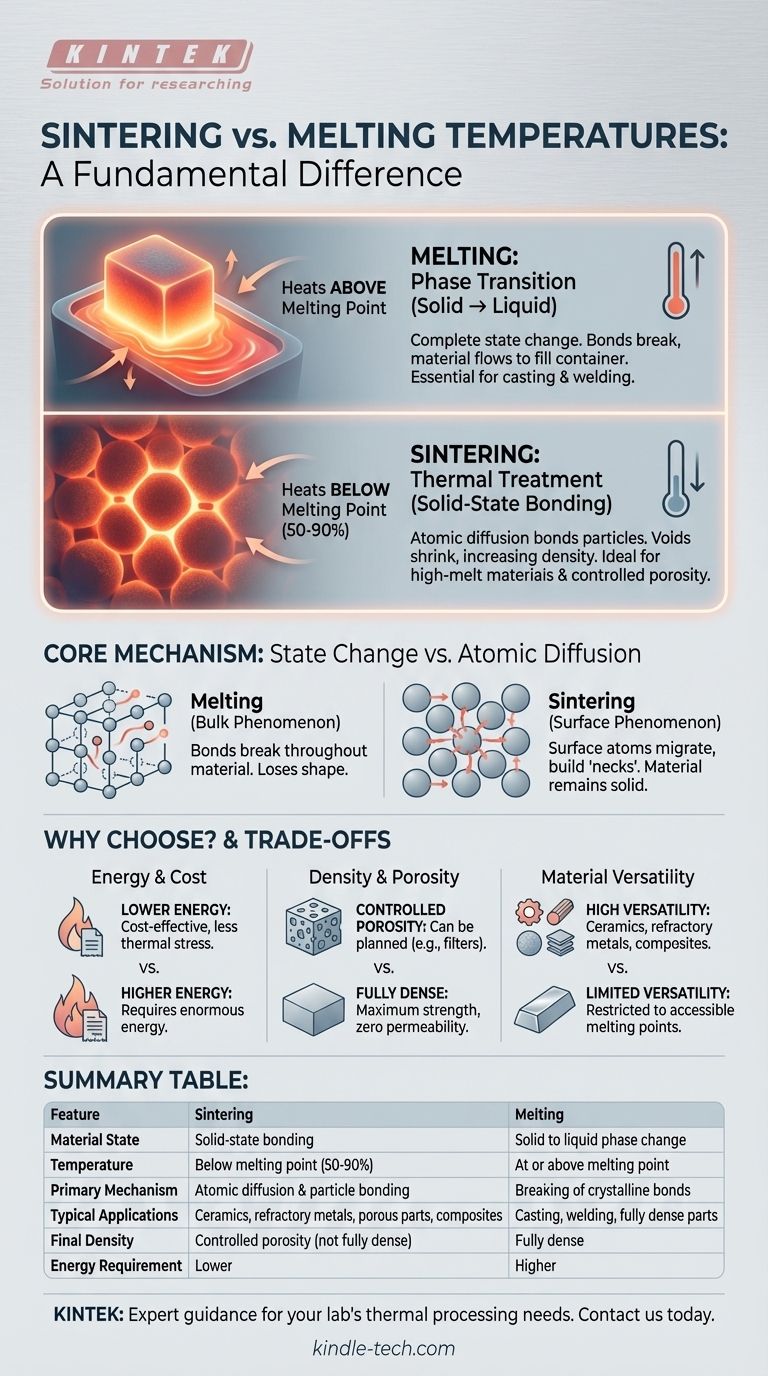
The fundamental difference between sintering and melting is the state of the material. Melting is a phase transition that turns a solid completely into a liquid by heating it above its melting point. Sintering, in contrast, is a thermal treatment that heats a compacted powder to a temperature below its melting point, causing the particles to fuse together without ever becoming fully liquid.
While both processes use heat to form a solid object, melting relies on a complete state change from solid to liquid. Sintering is a more subtle solid-state process that uses atomic diffusion to bond particles, enabling the fabrication of materials that are difficult or impossible to melt.

The Core Mechanism: State Change vs. Atomic Diffusion
At a microscopic level, these two processes operate on entirely different principles. Understanding this distinction is key to choosing the right manufacturing technique.
What Happens During Melting?
Melting is a bulk phenomenon. When a material reaches its specific melting temperature, the thermal energy is sufficient to break the ordered, crystalline bonds holding its atoms in a fixed lattice.
The entire material undergoes a phase transition from solid to liquid. It loses its shape and will flow to fill the container it's in, a process essential for casting and welding.
How Does Sintering Work?
Sintering is a surface phenomenon driven by atomic diffusion. A compacted powder is heated to a sintering temperature, typically 50-90% of the material's absolute melting point.
At this elevated temperature, the atoms on the surfaces of the individual powder particles become highly agitated. This allows them to migrate across the boundaries between particles, effectively building "necks" or bridges that weld the particles together.
The material as a whole never liquefies. Instead, the voids between particles gradually shrink, increasing the density and strength of the final part.
Why Choose One Process Over the Other?
The choice between sintering and melting is not about which is "better," but which is the right tool for a specific material and application.
When Sintering is the Superior Choice
Sintering is indispensable for materials with extremely high melting points, such as ceramics, tungsten, and molybdenum. Melting these materials requires enormous energy and specialized equipment.
It is also the go-to process for creating parts with controlled porosity, like filters or self-lubricating bearings. Because the process starts with particles, the final density can be precisely managed.
Finally, sintering allows for the creation of metal matrix composites by mixing powders of different materials (e.g., a metal and a ceramic) that could not be combined via melting due to vastly different melting points.
When Melting is Necessary
Melting is the required process for traditional casting. To create a fully dense part by pouring material into a mold, the material must be in a completely liquid state to fill every detail of the cavity.
It is also the basis for most welding techniques, where a localized pool of molten material is used to fuse two components together, creating a seamless, fully dense joint upon cooling.
Understanding the Trade-offs
Each process comes with a distinct set of advantages and limitations that directly impact cost, performance, and material selection.
Energy and Cost
Sintering almost always requires less energy than melting. Operating at a lower temperature directly translates to lower energy bills and less thermal stress on equipment, often resulting in a more cost-effective process.
Final Part Density and Porosity
Melting inherently produces a fully dense part (assuming no gas is trapped during cooling). This is ideal for applications requiring maximum strength and zero permeability.
Sintered parts, by contrast, almost always contain some level of residual porosity. While this can be a planned feature, it can also be a point of mechanical weakness if not properly controlled.
Material Versatility
Sintering opens the door to processing a wide range of refractory metals and ceramics that are impractical to melt. Its ability to combine disparate materials into composites is a unique advantage.
Melting is generally restricted to materials with more accessible melting points and cannot be used to create composites from materials that don't mix in a liquid state.
Making the Right Choice for Your Application
Selecting the correct thermal process depends entirely on your material constraints and the desired properties of the final component.
- If your primary focus is creating a fully dense, non-porous part from a conventional metal alloy: Melting through casting or welding is the most direct and reliable method.
- If your primary focus is working with high-temperature ceramics or refractory metals like tungsten: Sintering is the more practical, energy-efficient, and often the only viable manufacturing process.
- If your primary focus is creating a component with specific properties like controlled porosity or a composite structure: Sintering provides unique capabilities that melting cannot replicate.
Ultimately, choosing between these processes requires a clear understanding of your end goal, as each method transforms a raw material into a final part in a fundamentally different way.
Summary Table:
| Feature | Sintering | Melting |
|---|---|---|
| Material State | Solid-state bonding | Solid to liquid phase change |
| Temperature | Below melting point (50-90%) | At or above melting point |
| Primary Mechanism | Atomic diffusion & particle bonding | Breaking of crystalline bonds |
| Typical Applications | Ceramics, refractory metals, porous parts, composites | Casting, welding, fully dense parts |
| Final Density | Controlled porosity (not fully dense) | Fully dense |
| Energy Requirement | Lower | Higher |
Need expert guidance on selecting the right thermal process for your lab's materials?
At KINTEK, we specialize in providing high-quality lab equipment and consumables tailored to your specific sintering and melting needs. Whether you're working with high-temperature ceramics, refractory metals, or complex composites, our solutions ensure precise temperature control and reliable performance.
Contact us today to discuss how our expertise can help you optimize your thermal processing and achieve superior results in your laboratory.
Visual Guide
Related Products
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
- Molybdenum Vacuum Heat Treat Furnace
- 1700℃ Muffle Oven Furnace for Laboratory
People Also Ask
- How do you clean a tube furnace tube? A Step-by-Step Guide to Safe and Effective Cleaning
- What is the ceramic tube high temperature? From 1100°C to 1800°C, Choose the Right Material
- Why is an Alumina Ceramic Tube Support Necessary for 1100°C Experiments? Ensure Data Accuracy and Chemical Inertness
- What are the advantages of using an alumina liner in a tube furnace for biomass combustion corrosion simulations?
- What is the pressure on a tube furnace? Essential Safety Limits for Your Lab



















