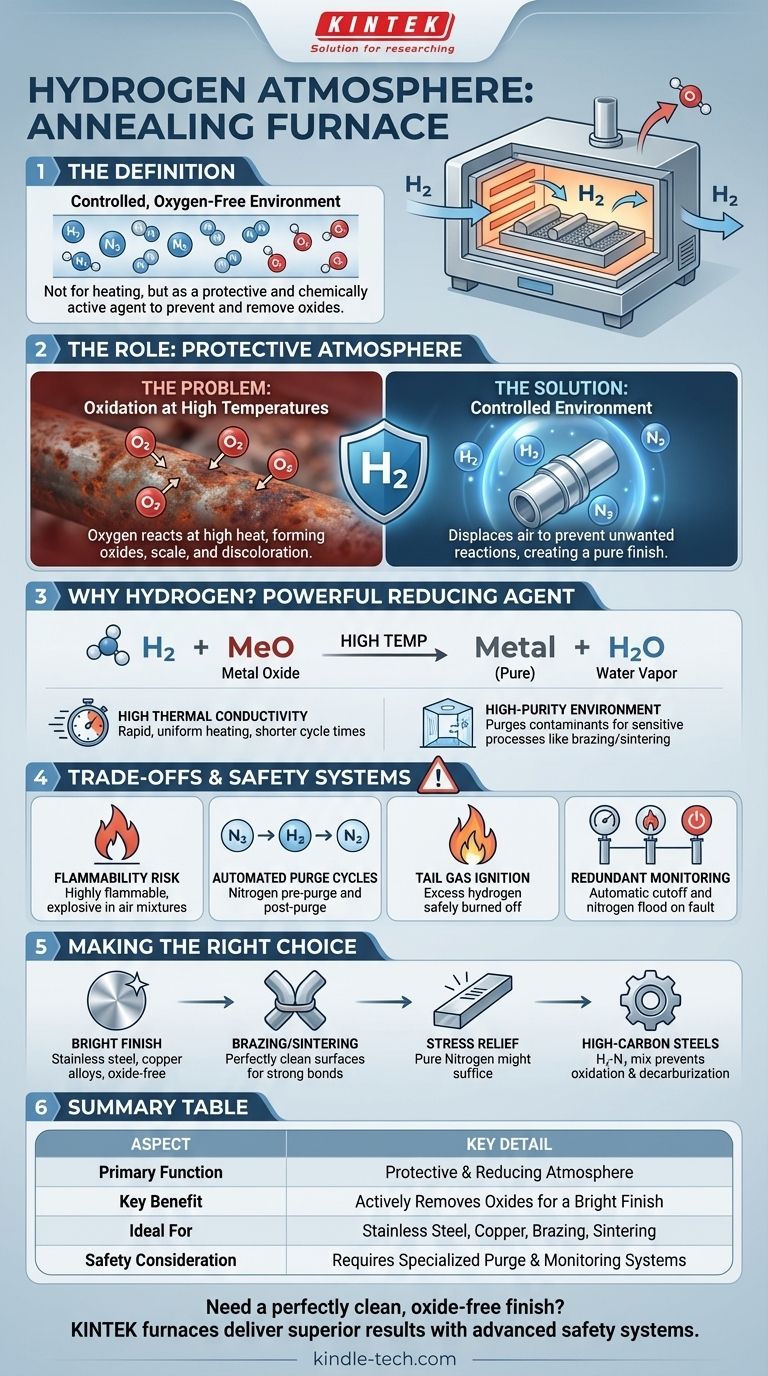
In short, a hydrogen atmosphere for an annealing furnace is a controlled, oxygen-free environment created by filling the furnace chamber with pure hydrogen gas or a hydrogen-nitrogen mixture. This specialized atmosphere is not used for heating itself, but to act as a protective and chemically active agent during the high-temperature treatment of materials. Its primary purpose is to prevent oxidation and to actively remove existing oxides from the material's surface.
The critical takeaway is that a hydrogen atmosphere does more than just protect a material from oxygen—it is a powerful reducing agent. Unlike inert gases that simply prevent new oxidation, hydrogen actively strips oxygen atoms from existing surface oxides, resulting in an exceptionally clean, bright, and metallurgically pure finish that is impossible to achieve in air or less reactive atmospheres.

The Role of a Protective Atmosphere
The Problem: Oxidation at High Temperatures
When metals are heated to annealing temperatures, their reactivity increases dramatically. Any oxygen present in the furnace, even in trace amounts from the air, will rapidly bond with the metal to form oxides, resulting in discoloration, scale, and compromised material properties.
The Solution: A Controlled Environment
A protective atmosphere displaces the ambient air inside the furnace, creating an environment that prevents these unwanted chemical reactions. While some atmospheres are inert (like pure nitrogen or argon), others are chemically active.
Why Hydrogen is the Atmosphere of Choice
As a Powerful Reducing Agent
The principal advantage of hydrogen is its ability to act as a strong reducing agent. At high temperatures, hydrogen molecules (H₂) react with metal oxides (MeO) on the material's surface, stripping away the oxygen to form water vapor (H₂O) and leaving behind pure metal.
This chemical cleaning action is essential for applications requiring a "bright" finish, free of any oxides.
High Thermal Conductivity
Hydrogen has a much higher thermal conductivity than air or nitrogen. This property allows for more rapid and uniform heating of the parts within the furnace, which can shorten cycle times and improve temperature consistency across the entire workload.
Creating a High-Purity Environment
Using a high-purity hydrogen or hydrogen-nitrogen mix effectively purges the furnace chamber of oxygen and other potential contaminants. This ensures that the material is processed in an exceptionally clean environment, which is critical for sensitive processes like brazing or sintering.
Understanding the Trade-offs and Safety Systems
The Inherent Risk of Flammability
The primary trade-off when using hydrogen is safety. Hydrogen is highly flammable and can be explosive when mixed with air within a specific concentration range. Therefore, hydrogen furnaces are engineered with multiple, redundant safety systems that are non-negotiable for safe operation.
Automated Purge Cycles
Before hydrogen is introduced, the furnace chamber is first purged with an inert gas, typically nitrogen, to remove all the oxygen from the air. Similarly, at the end of the cycle, the furnace is purged again with nitrogen to remove all hydrogen before the door can be opened.
Tail Gas Ignition
Excess hydrogen vented from the furnace cannot be released directly into the facility. It is piped to a "tail gas" port where it is automatically ignited and burned off safely. Flame detectors monitor this process continuously.
Redundant Monitoring and Shutdowns
Hydrogen furnaces are equipped with pressure sensors, flow controllers, and flame detectors. If the system detects a loss of flame at the burn-off port, a loss of pressure, or any other fault condition, it will automatically cut off the hydrogen supply, flood the furnace with nitrogen, and trigger an alarm.
Making the Right Choice for Your Application
The decision to use a hydrogen atmosphere depends entirely on your material and the desired outcome of the heat treatment process.
- If your primary focus is achieving a bright, oxide-free finish on materials like stainless steel or copper alloys: A hydrogen or hydrogen-nitrogen atmosphere is the ideal choice for its powerful reducing capabilities.
- If your primary focus is brazing or sintering metal components: A high-purity hydrogen atmosphere is often required to ensure surfaces are perfectly clean for strong, void-free metallurgical bonds.
- If your primary focus is simple stress-relief or recrystallization without strict surface requirements: A less expensive and safer atmosphere, such as pure nitrogen or an exothermic gas, may be sufficient.
- If your primary focus is treating high-carbon steels: A carefully controlled hydrogen-nitrogen mixture is often used to prevent not only oxidation but also surface decarburization.
Ultimately, a hydrogen atmosphere provides an unparalleled level of control over surface chemistry, enabling superior results for the most demanding thermal processes.
Summary Table:
| Aspect | Key Detail |
|---|---|
| Primary Function | Protective and reducing atmosphere |
| Key Benefit | Actively removes oxides for a bright finish |
| Ideal For | Stainless steel, copper alloys, brazing, sintering |
| Safety Consideration | Requires specialized purge and monitoring systems |
Need a perfectly clean, oxide-free finish for your materials? A hydrogen atmosphere furnace from KINTEK delivers the superior surface quality and metallurgical purity your laboratory requires. Our furnaces are engineered with advanced safety systems for reliable, high-performance results. Contact our thermal processing experts today to discuss your application!
Visual Guide
Related Products
- Graphite Vacuum Furnace Negative Material Graphitization Furnace
- 1200℃ Muffle Furnace Oven for Laboratory
- Horizontal High Temperature Graphite Vacuum Graphitization Furnace
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
People Also Ask
- What are the disadvantages of biomass conversion? High Costs, Logistical Hurdles, and Environmental Trade-offs
- What is the sputtering voltage of a magnetron? Optimize Your Thin Film Deposition Process
- What function does a high-temperature sintering furnace serve in biomass carbonization? Unlock Superior MFC Performance
- What is the significance of using a tube furnace with vacuum-sealed quartz tubes? Master Ceramic Synthesis
- What is the difference between oxidizing and reducing environments? Key Insights for Chemical Reactions















