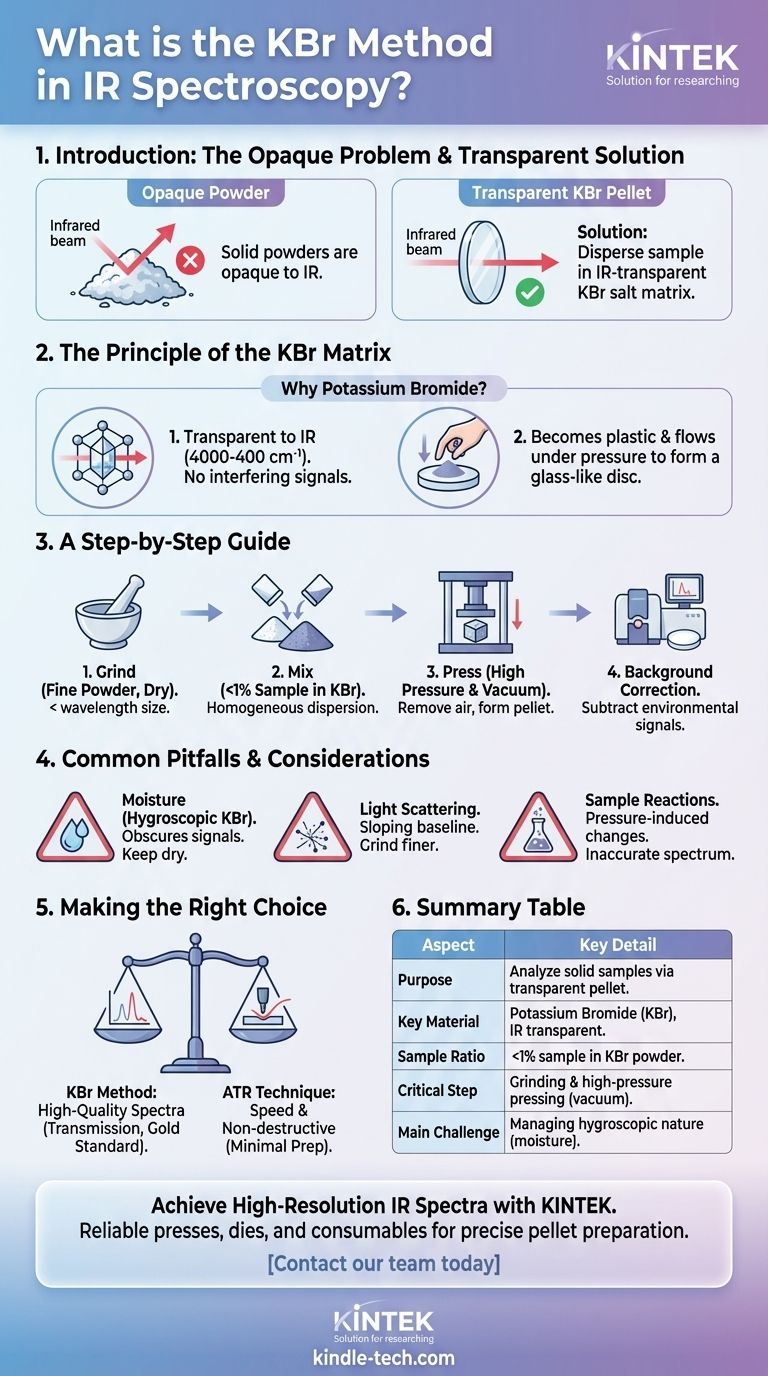
In infrared (IR) spectroscopy, the KBr method is a sample preparation technique used to analyze solid materials. It involves finely grinding a small amount of the sample with potassium bromide (KBr) powder and compressing the mixture under high pressure to form a thin, transparent pellet. This pellet can then be placed directly in the path of the spectrometer's infrared beam for analysis.
The core challenge in analyzing solid powders with IR spectroscopy is that they are typically opaque. The KBr method solves this by dispersing the sample within an IR-transparent salt matrix, effectively turning the powder into a "window" that infrared light can pass through.

The Principle of the KBr Matrix
Why Potassium Bromide?
Potassium bromide (KBr) is the standard choice for this technique for two critical reasons. First, it is transparent to infrared radiation across the most common analytical range (4000-400 cm⁻¹), meaning it doesn't produce interfering signals of its own.
Second, KBr and other alkali halides are unique in that they become plastic and flow under immense pressure. This property allows the fine powder to fuse together into a solid, glass-like disc that holds the sample in place.
The Goal: Homogeneous Dispersion
The ultimate goal of the method is to create a pellet where the microscopic particles of the sample are evenly and thinly distributed throughout the KBr matrix.
Proper dispersion minimizes light scattering and ensures the infrared beam interacts with a representative amount of the sample, yielding a clear and accurate spectrum.
A Step-by-Step Guide to the Method
Step 1: Grinding the Sample
The sample and the KBr must both be extremely dry and finely pulverized to achieve a good result. Typically, an agate mortar and pestle are used to grind the sample into a powder with a particle size smaller than the wavelength of the IR radiation to reduce scattering.
Step 2: Mixing
A very small amount of the ground sample (typically less than 1%) is added to a larger quantity of dry KBr powder. The two are mixed and ground together thoroughly to ensure the sample is homogeneously dispersed.
Step 3: Pressing the Pellet
The mixture is placed into a die and put into a hydraulic press. A vacuum is often applied to the die to remove trapped air and moisture, which can cause the pellet to be opaque or introduce spectral interference.
The press applies several tons of force, causing the KBr to plasticize and form a transparent pellet containing the sample.
Step 4: Background Correction
Before analyzing the sample, a background spectrum is run. This can be done with an empty pellet holder or, ideally, with a "blank" pellet made of pure KBr. This step allows the instrument's software to subtract any signals from atmospheric CO₂, water vapor, or impurities in the KBr itself.
Common Pitfalls and Considerations
The Challenge of Moisture
KBr is hygroscopic, meaning it readily absorbs moisture from the atmosphere. Water has very strong absorption bands in the infrared spectrum, which can easily obscure important signals from your sample.
All equipment, including the KBr powder and the sample, must be kept scrupulously dry, often by storing them in an oven or desiccator.
Light Scattering Effects
If the sample particles are not ground finely enough, they will scatter the infrared beam rather than absorbing it. This results in a poor-quality spectrum with a sloping baseline and reduced signal intensity, making interpretation difficult.
Potential for Sample Reactions
The high pressure used to form the pellet can sometimes induce chemical or physical changes in the sample. Certain compounds may react with the KBr itself, leading to an inaccurate spectrum that does not represent the original material.
Making the Right Choice for Your Goal
The KBr method is a powerful, classic technique, but modern alternatives exist. Your choice depends entirely on your analytical needs.
- If your primary focus is high-quality, high-resolution spectra: The KBr method, when executed carefully, is a gold standard for transmission measurements of solids.
- If your primary focus is speed and non-destructive analysis: A technique like Attenuated Total Reflectance (ATR) is often superior, as it requires almost no sample preparation.
- If your sample is sensitive to moisture or pressure: You must either take extreme precautions with the KBr method or choose an alternative technique to avoid artifacts.
Understanding the principles of the KBr method empowers you to generate clean, reliable spectral data from solid samples.
Summary Table:
| Aspect | Key Detail |
|---|---|
| Purpose | Analyze solid samples in IR spectroscopy by creating a transparent pellet. |
| Key Material | Potassium Bromide (KBr), transparent to IR light. |
| Sample Ratio | Typically <1% sample dispersed in KBr powder. |
| Critical Step | Grinding to a fine powder and applying high pressure with a vacuum. |
| Main Challenge | Managing KBr's hygroscopic nature to avoid moisture interference. |
Ready to achieve high-resolution IR spectra from your solid samples? The KBr method is a foundational technique, and having the right equipment is crucial for success. KINTEK specializes in providing reliable laboratory presses, dies, and consumables for precise pellet preparation.
Our experts can help you select the perfect tools to minimize moisture issues and ensure homogeneous pellets for clear, accurate results.
Contact our team today to discuss your lab's needs and enhance your IR spectroscopy capabilities!
Visual Guide
Related Products
- kbr pellet press 2t
- Laboratory Hydraulic Press Split Electric Lab Pellet Press
- Automatic Laboratory Hydraulic Press for XRF & KBR Pellet Press
- XRF & KBR steel ring lab Powder Pellet Pressing Mold for FTIR
- Automatic Laboratory Hydraulic Pellet Press Machine for Lab Use
People Also Ask
- What are the different types of sampling techniques used in IR spectroscopy? A Guide to KBr, Mull, and ATR Methods
- What are the safety precautions for KBr? Achieve Flawless FTIR Pellet Preparation and Data Accuracy
- How much sample is needed for IR? Optimize Your Analysis with Minimal Material
- How do you prepare a KBr pellet for IR spectroscopy? Master the Key Steps for a Clear Spectrum
- Why only KBr is used in IR spectroscopy? The Truth About the Best Material for Your Sample



















