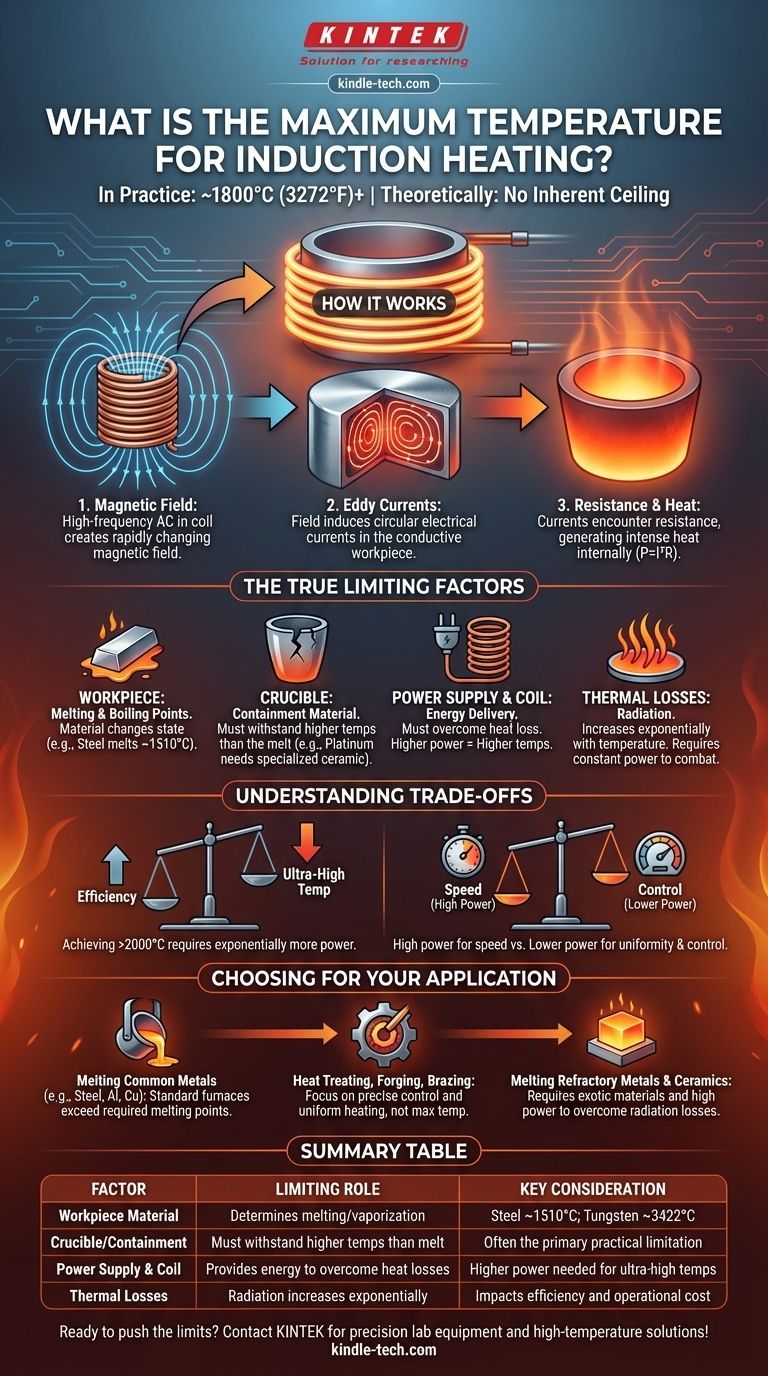
In practice, induction heating systems can readily achieve temperatures of 1800°C (3272°F) and significantly higher. However, the theoretical maximum temperature is not a limit of the induction process itself, but is instead determined by the physical properties of the material being heated and the equipment containing it.
The core principle to understand is that induction heating has no inherent temperature ceiling. The practical limits are imposed by the melting and vaporization points of the workpiece and the heat resistance of the crucible or surrounding components.

How Induction Heating Generates Extreme Temperatures
To understand the temperature limits, we must first understand the mechanism. The process relies on fundamental electromagnetic principles to generate heat directly within a material.
The Role of the Magnetic Field
An induction system uses a copper coil through which a high-frequency alternating current (AC) is passed. This creates a powerful and rapidly changing magnetic field around the coil.
Generating Eddy Currents in the Workpiece
When an electrically conductive material, or workpiece, is placed inside this magnetic field, the field induces circular electrical currents within it. These are known as eddy currents.
Resistance Creates the Heat
As these eddy currents flow through the material, they encounter electrical resistance. This resistance causes intense localized heating, a phenomenon described by the formula P = I²R (Power = Current² x Resistance). The heat is generated inside the part itself, not from an external flame or element.
The True Limiting Factors of Temperature
While the process is elegant, reaching and maintaining ultra-high temperatures is a battle against the laws of physics and material science. The "maximum temperature" is a function of overcoming these four key constraints.
The Workpiece's Melting and Boiling Point
The most obvious limit is the material itself. You can heat a piece of steel until it melts (around 1510°C) and then vaporizes (around 2862°C). The induction process can supply the energy to do this, but the material will change state, which is often the goal in a furnace application.
The Crucible or Containment Material
For melting applications, the workpiece is held in a container called a crucible. This crucible must remain solid at temperatures that exceed the melting point of the material inside it. For example, melting platinum (~1770°C) requires a specialized ceramic or graphite crucible that can withstand such extreme heat. The crucible is often the primary practical limitation.
Power Supply and Coil Design
The amount of energy delivered to the workpiece is determined by the power supply's output and the inductive coupling—the efficiency of the magnetic field transfer between the coil and the part. To reach higher temperatures, the system must pump in energy faster than it is lost to the environment. This requires more power and optimized coil design.
Thermal Losses to the Environment
As an object gets hotter, it radiates heat away more quickly. At extreme temperatures, this radiation becomes the dominant form of heat loss. An induction system must have enough power to constantly overcome these massive thermal losses to continue raising or even just maintaining the temperature.
Understanding the Trade-offs
Choosing or designing an induction system involves balancing competing factors. It is not simply about chasing the highest possible temperature.
Efficiency vs. Temperature
Achieving ultra-high temperatures (above 2000°C) requires exponentially more power to combat radiation losses. The system becomes less energy-efficient as the target temperature climbs, increasing operational costs significantly.
Material Constraints vs. Desired Goal
The material you need to heat dictates the entire system design. If you need to melt tungsten (melting point ~3422°C), your primary challenge is not the induction process, but sourcing crucible materials and insulators that can survive those conditions.
Speed vs. Control
A very high-power system can reach a target temperature almost instantly. However, this can cause thermal shock, damaging the workpiece. Lower power provides slower, more uniform heating and gives finer control, which is critical for applications like hardening and tempering.
Making the Right Choice for Your Application
Your application, not the technology's theoretical limit, should guide your decision.
- If your primary focus is melting common metals (e.g., steel, aluminum, copper): Standard induction furnaces are more than capable, as their operating temperatures easily and efficiently exceed the required melting points.
- If your primary focus is heat treating, forging, or brazing: The maximum temperature is far less important than precise temperature control and uniform heating, which are key strengths of induction.
- If your primary focus is melting refractory metals or advanced ceramics: Your project's success will depend on sourcing exotic containment materials and designing a system with sufficient power to overcome extreme thermal radiation losses.
Ultimately, the temperature you can achieve is a direct function of your system's design and the fundamental properties of your materials.
Summary Table:
| Factor | Limiting Role | Key Consideration |
|---|---|---|
| Workpiece Material | Determines melting/vaporization point | Steel melts at ~1510°C; Tungsten at ~3422°C |
| Crucible/Containment | Must withstand higher temps than the melt | Often the primary practical limitation |
| Power Supply & Coil | Provides energy to overcome heat losses | Higher power needed for ultra-high temperatures |
| Thermal Losses | Radiation increases exponentially with temperature | Impacts efficiency and operational cost |
Ready to push the limits of high-temperature processing? KINTEK specializes in precision lab equipment and consumables for demanding applications. Whether you're melting refractory metals or require precise heat treatment, our expertise in induction heating systems and high-temperature materials ensures your lab operates at peak performance. Contact our experts today to discuss your specific temperature and material challenges!
Visual Guide
Related Products
- Lab-Scale Vacuum Induction Melting Furnace
- 600T Vacuum Induction Hot Press Furnace for Heat Treat and Sintering
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- Ultra-High Temperature Graphite Vacuum Graphitization Furnace
People Also Ask
- What is vacuum arc melting technique? Discover the Precision of Vacuum Induction Melting
- What is the difference between induction melting and vacuum induction melting? Choosing the Right Process for Purity
- What types of metals are typically processed in a vacuum induction melting furnace? High-Purity Alloys for Critical Applications
- What is the principle of vacuum induction melting? Achieve Ultra-High Purity Metals
- What is the primary function of a vacuum induction melting furnace? Melt High-Purity Metals with Precision



















