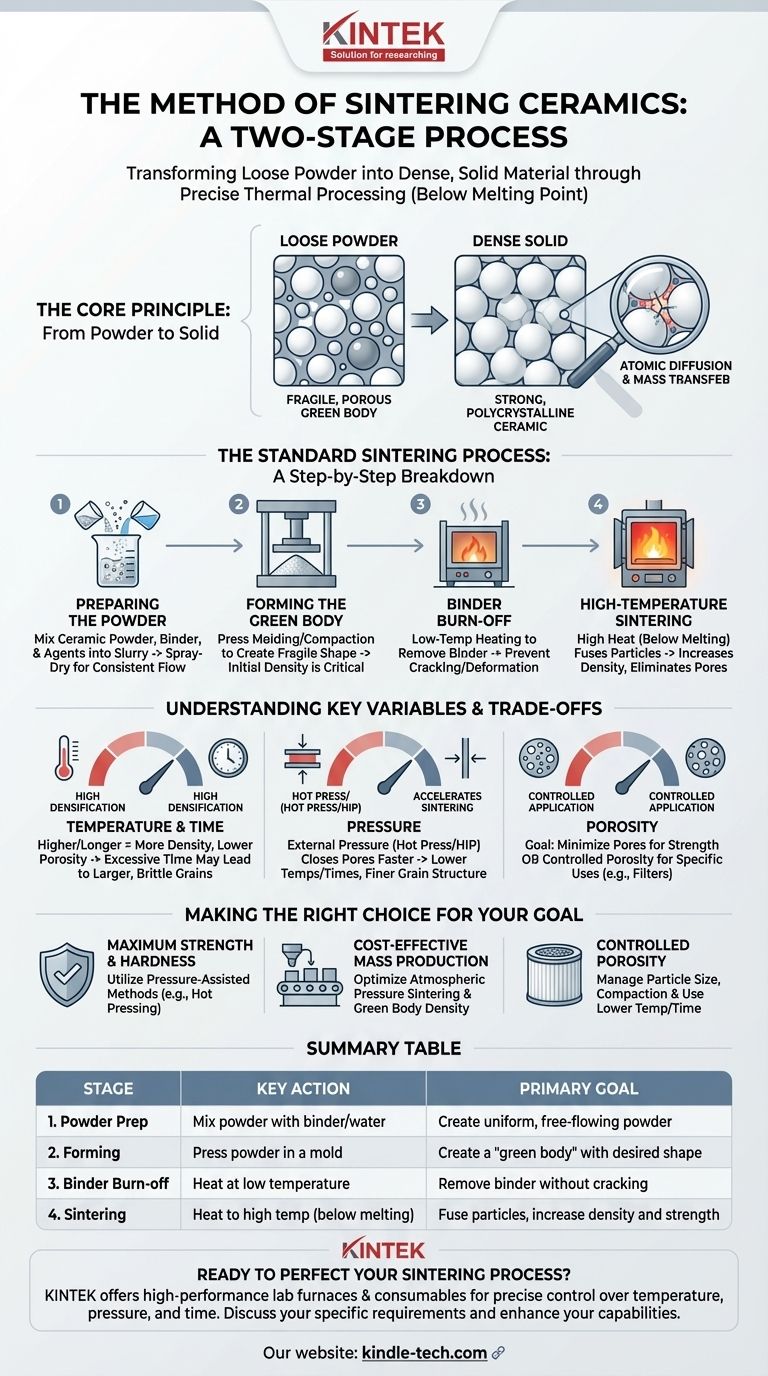
At its core, the method of sintering ceramics is a two-stage process. First, a ceramic powder is mixed with a binder and compressed into a desired shape, known as a "green body." This fragile object is then heated to a very high temperature, causing the individual ceramic particles to fuse together into a dense, hard, and solid final product.
Sintering is not merely heating; it is a precise thermal process that transforms a loose powder compact into a dense polycrystalline material. The fundamental goal is to eliminate the pores between particles, creating a strong, unified microstructure with specific mechanical and thermal properties.

The Core Principle: From Powder to Solid
What Happens During Sintering?
Sintering is a process of densification driven by high temperature, but crucially, this temperature is below the material's melting point. Instead of melting into a liquid, the atoms on the surface of the ceramic particles become mobile.
This atomic movement causes the particles to bond and fuse at their points of contact. Think of it like a bucket of packed snowballs left in a cold environment; over time, the individual snowballs will fuse into a solid block of ice without ever melting into water.
The Microscopic Transformation
As the process continues, mass transfers from the particles to fill the voids (or pores) between them. This results in the overall object shrinking in volume and increasing in density.
The end result is a polycrystalline ceramic, a solid material made of many tiny, interlocked crystalline grains. The final properties, such as strength and hardness, are determined by the size of these grains and the amount of remaining porosity.
The Standard Sintering Process: A Step-by-Step Breakdown
Step 1: Preparing the Powder
The journey begins with a raw ceramic powder. This powder is often mixed with water, a binder (a type of glue to hold the shape), and other agents to form a uniform, liquid-like mixture called a slurry.
This slurry is then typically spray-dried to create a consistent, free-flowing powder that is ideal for pressing.
Step 2: Forming the Green Body
The prepared powder is placed into a mold and subjected to high pressure. This step, known as press molding or compaction, forms the powder into a fragile, preliminary shape called a green body.
The green body has the desired geometry but lacks any significant strength. Its initial density is a critical factor, as it directly influences the final porosity of the sintered part.
Step 3: Binder Burn-off
Before the final high-temperature firing, the green body undergoes a lower-temperature heating cycle. The purpose of this step is to slowly and carefully burn off the binder added in Step 1.
Rushing this stage can cause the part to crack or deform as the binder gases escape too quickly.
Step 4: High-Temperature Sintering
This is the final and most critical stage. The green body is heated in a furnace to an extreme temperature, often for several hours. During this time, the atomic diffusion and mass transfer occur, fusing the particles, eliminating porosity, and causing the part to densify and shrink.
The precise temperature and duration are carefully controlled to achieve the desired microstructure and final properties.
Understanding the Trade-offs and Key Variables
The Role of Temperature and Time
Higher temperatures and longer sintering times generally lead to greater densification and lower porosity. However, this also causes the crystalline grains to grow larger.
Excessive grain growth can sometimes be detrimental, making the ceramic more brittle. There is a delicate balance between achieving high density and controlling the final grain size.
The Impact of Pressure
Applying external pressure during heating, as in hot pressing or hot isostatic pressing (HIP), dramatically accelerates the densification process.
Pressure helps close pores more effectively, allowing for sintering to occur at lower temperatures or in shorter times. This often results in a final product with superior density and a finer grain structure.
Controlling Final Porosity
The final porosity is a direct result of the initial porosity of the green body and the sintering parameters. While the goal is often to eliminate pores for maximum strength, some applications, like ceramic filters, require a specific, controlled level of porosity.
For pure oxide ceramics, where atomic diffusion is slower, achieving low porosity requires very high temperatures or the assistance of pressure.
Making the Right Choice for Your Goal
Achieving the desired outcome in ceramic manufacturing requires tailoring the sintering process to the specific goal.
- If your primary focus is maximum strength and hardness: Utilize pressure-assisted methods like hot pressing to achieve near-full density and a fine-grained microstructure.
- If your primary focus is cost-effective mass production: Rely on conventional atmospheric pressure sintering, optimizing the green body density and firing cycle for an acceptable balance of properties and throughput.
- If your primary focus is creating a part with controlled porosity: Carefully manage the initial particle size, compaction pressure of the green body, and keep sintering temperatures and times lower to prevent full densification.
Ultimately, mastering sintering is about controlling heat, time, and pressure to transform a simple powder into a high-performance engineered material.
Summary Table:
| Stage | Key Action | Primary Goal |
|---|---|---|
| 1. Powder Prep | Mix powder with binder/water | Create uniform, free-flowing powder |
| 2. Forming | Press powder in a mold | Create a 'green body' with desired shape |
| 3. Binder Burn-off | Heat at low temperature | Remove binder without cracking the part |
| 4. Sintering | Heat to high temperature (below melting point) | Fuse particles, increase density and strength |
Ready to perfect your ceramic sintering process? The right lab equipment is crucial for controlling temperature, pressure, and time to achieve your desired material properties. At KINTEK, we specialize in high-performance lab furnaces and consumables designed for precise thermal processing. Whether you're focused on R&D or mass production, our solutions help you create stronger, denser ceramics efficiently.
Contact our experts today to discuss your specific sintering requirements and discover how KINTEK can enhance your laboratory's capabilities.
Visual Guide
Related Products
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1700℃ Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- Why is an Alumina Ceramic Tube Support Necessary for 1100°C Experiments? Ensure Data Accuracy and Chemical Inertness
- What is the pressure on a tube furnace? Essential Safety Limits for Your Lab
- What are the common applications for a tube furnace? Essential for Heat Treatment, Synthesis, and Purification
- What factors influence the general design of a tube furnace? Match Your Process with the Perfect System
- What tube is used for tubular furnace? Choose the Right Material for Temperature & Atmosphere



















