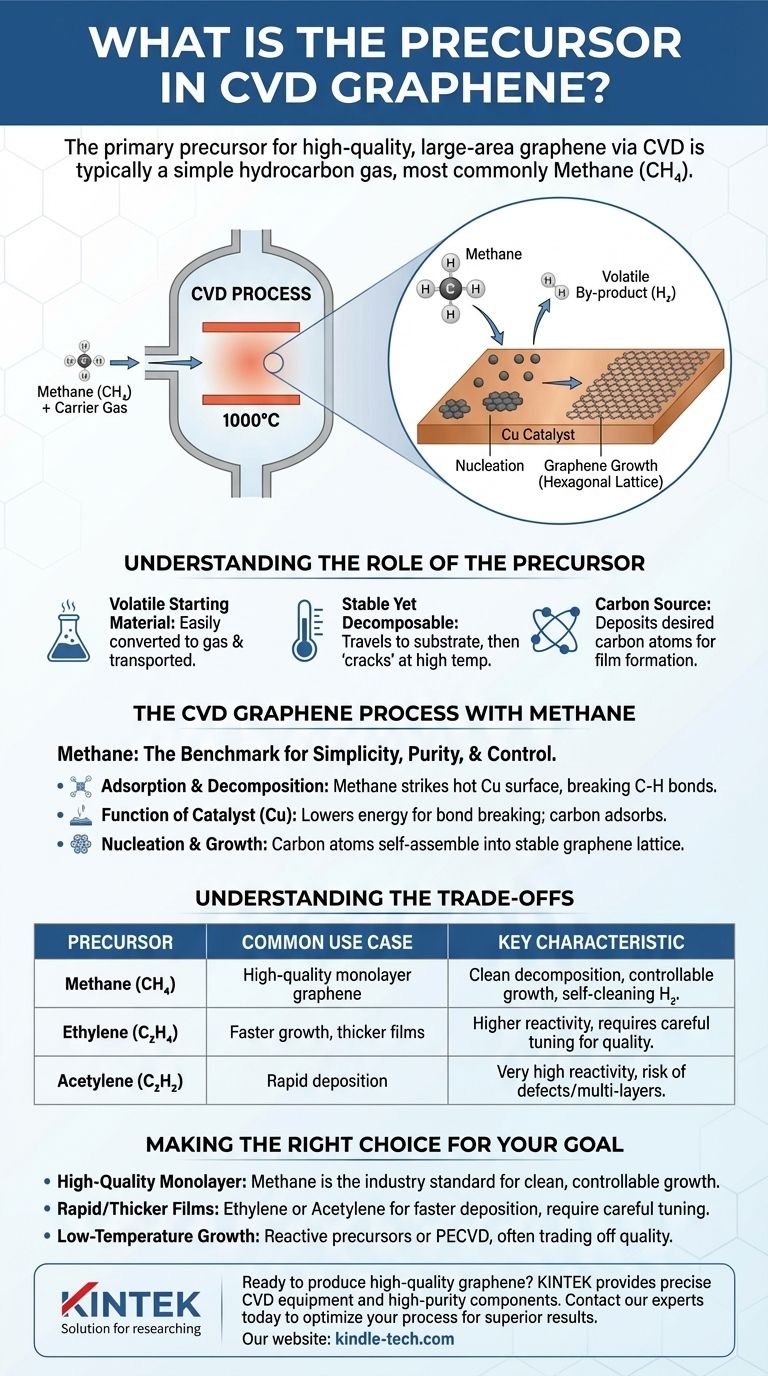
The primary precursor for producing high-quality, large-area graphene via Chemical Vapor Deposition (CVD) is typically a simple hydrocarbon gas, with methane (CH₄) being the most common and well-established choice. This precursor acts as the carbon source, which is chemically broken down at high temperatures to form a single atomic layer of graphene on a catalyst substrate.
The core principle of CVD graphene synthesis is not just finding a source of carbon, but selecting a volatile precursor that can be controllably decomposed. Methane is the standard because its simple structure allows for a clean reaction, depositing carbon atoms that self-assemble into high-quality graphene while the hydrogen by-products are easily removed.

Understanding the Role of the Precursor
In any CVD process, the precursor is the foundational ingredient. It is a chemical compound that contains the elements you wish to deposit as a thin film.
A Volatile Starting Material
A precursor must be volatile, meaning it can be easily converted into a gas and transported into the reaction chamber. However, it must also be stable enough to travel to the heated substrate without decomposing prematurely.
These gaseous precursor molecules are then introduced into a high-temperature reactor where the deposition will occur.
From Gas to Solid Film
Inside the reactor, the intense heat causes a chemical reaction on the surface of a substrate. The precursor molecules decompose, or "crack," depositing the desired element (in this case, carbon) onto the substrate while other elements are released as volatile by-products.
The CVD Graphene Process with Methane
Methane (CH₄) has become the benchmark precursor for graphene synthesis due to its simplicity, high purity, and predictable behavior.
Adsorption and Decomposition
The process typically occurs at temperatures around 1000 °C inside a quartz tube furnace. Methane gas is flowed over a catalytic substrate, most commonly a thin foil of copper (Cu). When the methane molecules strike the hot copper surface, they break apart.
The Function of the Catalyst
The copper catalyst is crucial. It lowers the energy required to break the carbon-hydrogen bonds in the methane molecules. The carbon atoms then adsorb onto or dissolve into the surface of the copper.
The hydrogen atoms, which are by-products of the reaction, are simply swept away in the gas flow.
Nucleation and Growth
As the carbon atoms accumulate on the copper surface, they begin to move and connect with each other. They self-assemble into the stable, hexagonal lattice structure of graphene, forming small islands that grow and eventually merge into a continuous, single-atom-thick sheet covering the substrate.
Understanding the Trade-offs
While methane is the standard, the choice of precursor has direct consequences on the final product and the complexity of the process.
Why Not Other Carbon Sources?
Other carbon-containing precursors, such as ethylene (C₂H₄) or acetylene (C₂H₂), can also be used. These molecules contain more carbon and can lead to faster growth rates.
However, their increased reactivity can make it more difficult to control the deposition, often resulting in the formation of multiple graphene layers (bilayer or few-layer graphene) or lower-quality films with more defects.
The Benefit of Hydrogen By-product
The hydrogen gas released from methane decomposition is not just a waste product. It can act as a mild etchant, helping to remove less stable, non-crystalline carbon formations (amorphous carbon) from the substrate. This "self-cleaning" effect contributes to the higher quality of graphene grown from methane.
The Challenge of Solid or Liquid Precursors
While solid and liquid carbon sources can be used, they add complexity. They must first be vaporized into a gaseous state before being introduced into the reactor, which requires additional equipment and precise temperature control to ensure a stable and repeatable flow rate.
Making the Right Choice for Your Goal
The ideal precursor is directly tied to the desired outcome of your synthesis process.
- If your primary focus is high-quality, large-area monolayer graphene: Methane is the industry-standard precursor due to its clean decomposition, controllable growth, and the beneficial etching effect of its hydrogen by-product.
- If your primary focus is rapid growth or thicker films: Ethylene or acetylene can offer faster deposition rates, but they require more careful process tuning to manage film quality and thickness.
- If your primary focus is low-temperature growth: Using more reactive precursors or plasma-enhanced CVD (PECVD) can enable deposition at lower temperatures, though this often involves a trade-off in film uniformity and quality.
Ultimately, mastering the interplay between the precursor, catalyst, and process conditions is the key to producing graphene tailored for any application.
Summary Table:
| Precursor | Common Use Case | Key Characteristic |
|---|---|---|
| Methane (CH₄) | High-quality monolayer graphene | Clean decomposition, controllable growth |
| Ethylene (C₂H₄) | Faster growth, thicker films | Higher reactivity, requires careful tuning |
| Acetylene (C₂H₂) | Rapid deposition | Very high reactivity, risk of defects |
Ready to produce high-quality graphene for your research or application? The choice of precursor is critical for achieving the desired film properties. KINTEK specializes in providing the precise lab equipment and consumables, including CVD systems and high-purity gas delivery components, needed to master your graphene synthesis process. Contact our experts today to discuss how we can support your laboratory's specific needs and help you optimize your CVD parameters for superior results.
Visual Guide
Related Products
- Microwave Plasma Chemical Vapor Deposition MPCVD Machine System Reactor for Lab and Diamond Growth
- Cylindrical Resonator MPCVD Machine System Reactor for Microwave Plasma Chemical Vapor Deposition and Lab Diamond Growth
- CVD Diamond Cutting Tool Blanks for Precision Machining
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
People Also Ask
- How does Microwave Plasma Chemical Vapour Deposition (MPCVD) work? Your Guide to High-Purity Diamond Film Growth
- How plasma is used in diamond coating films? Unlock the Power of MPCVD for Superior Coatings
- What is the frequency of MPCVD? A Guide to Choosing 2.45 GHz vs. 915 MHz for Your Application
- What are the limitations of diamonds? Beyond the Myth of Perfection
- What are the primary advantages of the CVD method for growing diamonds? Engineering High-Purity Gems and Components



















