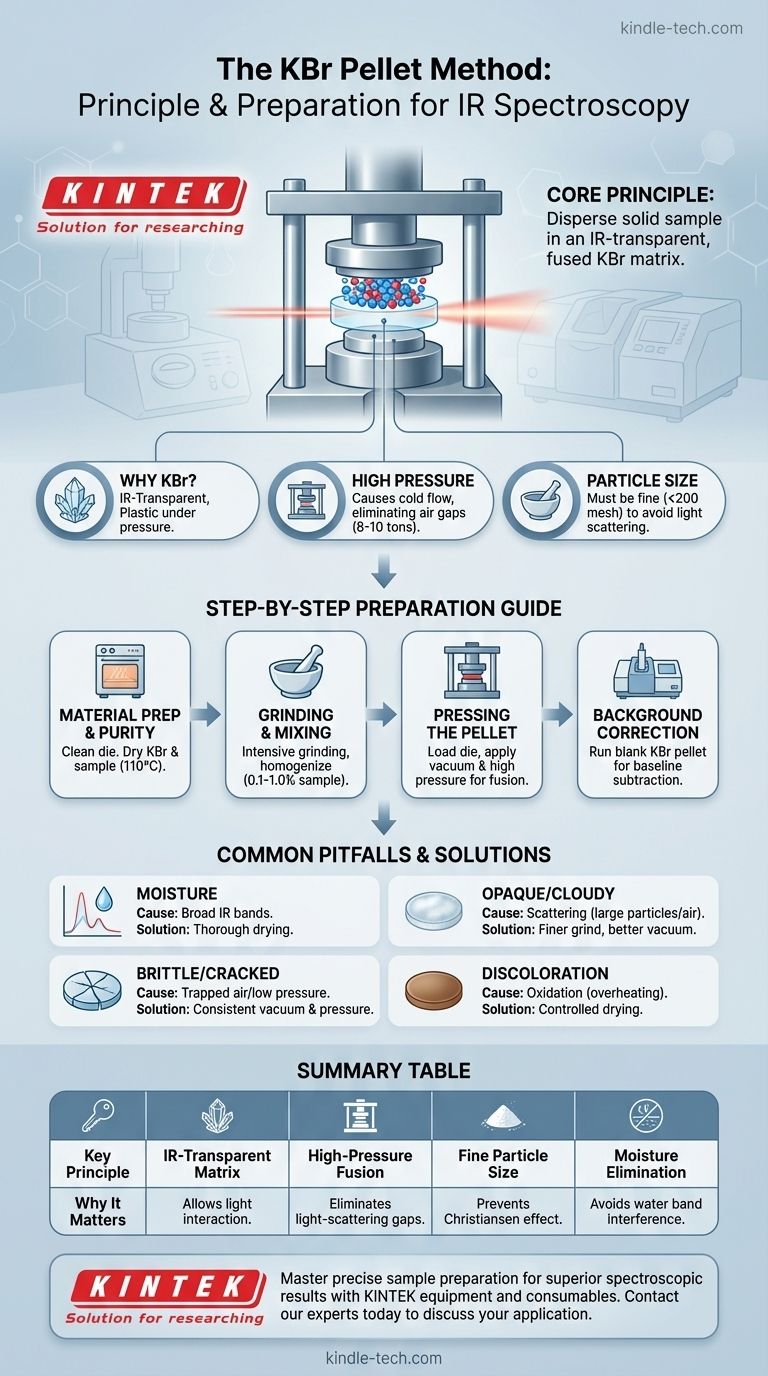
At its core, the principle of the KBr pellet method is to uniformly disperse a solid sample within an infrared-transparent matrix. Potassium bromide (KBr) is used because it is an alkali halide salt that becomes plastic under high pressure, fusing into a solid, glass-like disc that is transparent to infrared light. This allows for the spectroscopic analysis of solid samples that cannot be easily measured in their native state.
The KBr pellet method is a sample preparation technique for infrared spectroscopy that transforms a solid powder sample into a transparent medium. Success is not based on complex chemistry, but on the physical properties of KBr and meticulous preparation to eliminate moisture and ensure particle uniformity.

The Underlying Principle: Creating an "Invisible" Matrix
To understand the KBr method, you must first understand the material itself and the physics of the process. The goal is to make the KBr matrix "disappear" from the perspective of the spectrometer, leaving only the sample to be analyzed.
Why Potassium Bromide (KBr)?
KBr is the ideal medium for two primary reasons. First, it exhibits almost no absorption of light in the mid-infrared region, the range most commonly used for chemical analysis.
Second, KBr is a crystalline salt that deforms plastically under immense pressure. This causes the individual salt grains to fuse together, forming a homogenous, transparent sheet.
The Role of High Pressure
The application of high pressure (typically 8-10 tons) is what forces the KBr powder to "cold flow" and form a solid disc.
This process eliminates the air between the KBr particles, creating a solid matrix that encases the sample particles. This is crucial for preventing light from scattering, which would otherwise obscure the measurement.
The Importance of Particle Size
Both the sample and the KBr powder must be ground to a very fine, uniform particle size (typically below 200 mesh).
If particles are too large, they will scatter the infrared light instead of absorbing it, a phenomenon known as the Christiansen effect. This scattering appears as a broad, distorted baseline in the final spectrum, making interpretation difficult or impossible.
A Step-by-Step Guide to Pellet Preparation
The quality of your final spectrum is determined entirely by the quality of your preparation. Each step is designed to mitigate a potential problem.
Step 1: Material Preparation and Purity
Before beginning, all parts of the pellet die must be cleaned thoroughly to remove any contaminants from previous use.
The KBr powder must be spectroscopy-grade and perfectly dry. It should be heated in an oven at approximately 110°C for 2-3 hours to remove any adsorbed moisture, which has strong IR absorption bands. The sample itself should also be as dry as possible.
Step 2: Grinding and Mixing
A small amount of the solid sample (0.1% to 1.0% by weight) is added to the dried KBr powder.
The mixture is then ground intensively, typically with an agate mortar and pestle, to reduce the particle size and ensure the sample is homogenously distributed throughout the KBr.
Step 3: Pressing the Pellet
The ground mixture is loaded into the pellet die. The die is then placed in a hydraulic press and a vacuum is applied to remove any trapped air and residual moisture.
High pressure is applied to fuse the powder into a transparent or translucent disc. The resulting pellet is then carefully removed from the die.
Step 4: Background Correction
Before measuring the sample, it is best practice to run a background spectrum. This can be done with an empty sample holder or, ideally, with a "blank" pellet made of pure KBr.
This allows the instrument's software to subtract any minor signals from atmospheric CO2, water vapor, or the KBr matrix itself, isolating the true spectrum of your sample.
Understanding the Trade-offs and Common Pitfalls
The KBr method is powerful, but it is highly susceptible to procedural errors. Understanding these common failures is key to troubleshooting.
The Problem of Moisture
Moisture is the primary enemy of this technique. Water has very strong, broad absorption bands in the infrared spectrum that can easily overwhelm the signal from your sample. Inadequate drying of the KBr or sample is the most common cause of a poor-quality spectrum.
Opaque or Cloudy Pellets
A pellet that is not transparent is a sign of excessive light scattering. This is almost always caused by one of two issues: insufficient grinding (particles are too large) or an inadequate vacuum during pressing (trapped air creates imperfections).
Brittle or Cracked Pellets
Pellets that break easily are often the result of trapped air. Applying a strong, consistent vacuum during the pressing phase is critical to creating a mechanically stable disc. Insufficient pressure can also lead to a weak pellet.
Pellet Discoloration (Browning)
If KBr is heated too rapidly or at too high a temperature during the drying step, it can oxidize into potassium bromate (KBrO3). This can cause a yellow or brown discoloration in the pellet and introduce unwanted artifacts into the spectrum.
Making the Right Choice for Your Goal
Your success with the KBr method depends on your specific analytical goal. Focus your efforts on the parameters that matter most for your application.
- If your primary focus is qualitative identification: Your main goal is a clear, artifact-free pellet; prioritize thorough grinding and meticulous drying to get a recognizable spectral fingerprint.
- If your primary focus is quantitative analysis: Consistency is paramount; precisely control the sample-to-KBr ratio, pellet thickness, and pressing force for every standard and sample to ensure results are comparable.
- If you are troubleshooting a poor spectrum: Immediately suspect moisture contamination or inadequate grinding, as these two factors are the most common sources of spectral noise and distortion.
Mastering the KBr pellet technique is a matter of meticulous preparation, transforming a challenging solid sample into a clear window for analysis.
Summary Table:
| Key Principle | Why It Matters |
|---|---|
| IR-Transparent Matrix | KBr becomes transparent under pressure, allowing IR light to pass through and interact with the sample. |
| High-Pressure Fusion | Forces KBr particles to cold-flow and fuse, eliminating air gaps that cause light scattering. |
| Fine Particle Size | Prevents light scattering (Christiansen effect) for a clean, interpretable spectrum. |
| Moisture Elimination | Critical step to avoid strong water absorption bands that can overwhelm the sample signal. |
Master precise sample preparation for superior spectroscopic results.
The KBr pellet method is a foundational technique, but its success hinges on meticulous execution and reliable equipment. Whether your focus is qualitative identification or rigorous quantitative analysis, the right tools are essential for creating clear, artifact-free pellets every time.
KINTEK specializes in lab equipment and consumables, serving laboratory needs. We provide the high-quality presses, pellet dies, and spectroscopy-grade materials you need to achieve consistent, publication-ready results. Let our expertise help you eliminate common pitfalls like moisture contamination and inadequate pressing.
Contact our experts today to discuss your specific application and ensure your sample preparation is never the weak link in your analysis.
Visual Guide
Related Products
- Automatic Laboratory Hydraulic Pellet Press Machine for Lab Use
- kbr pellet press 2t
- Automatic Laboratory Hydraulic Press for XRF & KBR Pellet Press
- Laboratory Hydraulic Press Split Electric Lab Pellet Press
- Laboratory Hydraulic Press Lab Pellet Press for Button Battery
People Also Ask
- What is the function of a laboratory hydraulic press during the fabrication of Beta-Al2O3 solid electrolyte pellets?
- How does a laboratory hydraulic pellet press contribute to SiCw/2024 aluminum composite preforms? Optimize Densification
- What role does a laboratory hydraulic press play in the preparation of solid electrolyte pellets? Ensure Data Accuracy
- How do laboratory hydraulic presses facilitate biomass pelletization? Optimize Biofuel Density and Prevent Slagging
- Why is a hydraulic pellet press used for FTIR? Transform Nanofillers into Clear Data



















